Why Was Migraleve Taken Off The Market?

Migraleve is a combination analgesic medication principally containing Paracetamol (acetaminophen), and codeine. Migraleve is used for the treatment of migraine attacks, including the symptoms of migraine.
Migraine is a headache that can cause severe throbbing pain or a pulsing sensation, usually on one side of the head. It’s often accompanied by nausea, vomiting, and extreme sensitivity to light and sound.
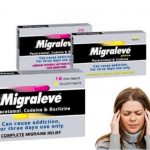
There are two variants of Migraleve: Yellow and pink, which both contain the analgesics Paracetamol and codeine. The yellow variant is designed to address the symptoms of headache and discomfort, whilst the pink variant also addresses symptoms of nausea and vomiting. A third Migraleve variant, Migraleve Ultra, contains sumatriptan.
Migraleve is classified in the UK as a pharmacy medication, available without prescription but more rigorously controlled than general sale list products, which can be sold in shops without pharmacist supervision.
Why was Migraleve taken off the market?
The scarcity of Migraleve began in 2018, due to the supply shortage issue with an ingredient in Migraleve pink tablets. A statement released by Johnson & Johnson read: “Unfortunately, Migraleve Pink, and Migraleve packs containing Pink Tablets and Yellow Tablets, are temporarily out of stock. We apologize to consumers for the inconvenience caused and would like to reassure them that we are working hard to resolve this situation. Migraleve Yellow remains unaffected.
Migraleve Pink tablets are intended to be taken upon the first presentation of migraine symptoms to fight pain and reduce nausea, while Migraleve Yellow is recommended as a follow-up if ongoing pain relief is needed. As the different tablet types are frequently taken in sequence, they are often sold together in a single package.
J&J’s social media channels are filled with complaints and inquiries about the missing Migraleve Pink tablets, with many users becoming increasingly frantic over their inability to access an over-the-counter treatment that some have described as “the only thing that works”.
Special Safety Warnings About Migraleve
■ Codeine is transformed to morphine in the liver by an enzyme. Morphine is the substance that produces pain relief. Some people have a variation of this enzyme and this can affect people in different ways. In some people, morphine is not produced or produced in very small quantities, and it will not provide enough pain relief. Other people are more likely to get serious side effects because a very high amount of morphine is produced. If you notice any of the following side effects, you must stop taking this medicine and seek immediate medical advice: slow or shallow breathing, confusion, sleepiness, small pupils, feeling or being sick, constipation, lack of appetite.
■ This medicine contains codeine which can cause addiction if you take it continuously for more than three days. If you need to use this medicine for more than three days at a time, see your doctor, pharmacist, or healthcare professional.
■ When you stop taking it you may feel restless and irritable.
■ Hyperalgesia may occur with the use of opioids or as a side effect during withdrawal, particularly with high doses. An unexplained increase in pain or increased levels of pain can occur with increasing opioid dosages. If you are on any opioid for pain, consult a physician before using this product.
■ Talk to your doctor or pharmacist if you have any breathing problems whilst sleeping.
■ Use in children and adolescents after surgery: Codeine should not be used for pain relief in children and adolescents after removal of their tonsils or adenoids for Obstructive Sleep Apnea Syndrome.
■ Use in children with breathing problems: Codeine is not recommended in children with breathing problems, since the symptoms of morphine toxicity may be worse in these children.
Driving and using machines
■ Migraleve Pink tablets may cause drowsiness. If affected, do not drive or operate machinery.