Drug Recall: Types, Reasons, What To Do, Recall Vs Withdrawal

According to the FDA, a recall is defined as an action executed by a manufacturer at any time to remove a defective or harmful drug product from the market when the drug is discovered to be in violation of laws and regulations administered by the FDA. A drug recall is the most effective means of protecting the public from a defective or potentially harmful product.
Approximately 80% of medications that Americans take contain some component manufactured abroad, primarily in China and India. While these manufacturing processes aid in keeping the costs of medications down, this has also created a supply chain that can be challenging to keep track of, which may increase the number of drug recalls. According to a recent report conducted by Kaiser Health News (KHN), thousands of drugs are recalled annually after reaching pharmacies; the recalls eventually impact patients when prescriptions are filled.
Why a recall is initiated?
A drug or medical product may be recalled for an assortment of reasons including:
- Hazardous Nature of Drug: More often than not, risks associated with the drug come to light once it has hit the market. For example, in 2000, a large number of drugs were called after they increased the risk of hemorrhagic in the brain due to the presence of Phenylpropanolamine(PPA). In such cases, Agencies request a recall of the drug from the market.
- Contaminated Ingredients: Sometimes, the manufacturing process of a drug may expose it to contamination by unwanted or harmful ingredients which may cause reactions to the end-user and turn out to be a health risk. In November 2018, a hypertension drug was recalled by the Food and Drugs Administration (FDA) due to contamination after routine ingredient testing detected high levels of N-nitrosoirbesartan, a potentially cancer-causing impurity.
- Defects in manufacturing: Errors or defects in the manufacturing process of a drug may result in compromised safety and efficiency of the drug product. The reason for a drug defect may vary from pharmacological variability between drug batches or between generic and originator drugs, incorrect drug quantity to the presence of impurities. If manufacturers are not aligned with Good Manufacturing Practice (GMP) guidelines of the respective Health Authority, they might be asked to recall the product.
- Errors in labeling: Labeling and packaging of a drug act as a guide for end-users. Any mistake in labeling or misleading message may lead to misinterpretation by the patients, which in turn leads to an adverse reaction. If a mistake is found in the packaging or labeling or, if it is not compliant with regulations, a recall is issued. Recently, a leading pharmaceutical company had to recall its fever medication for children due to mismatched instructions.
Apart from the above-mentioned, there might be many hidden reasons why a drug/device/cosmetic/food supplement is called off. Even though most drug recalls are a result of minor factors, it is necessary to prevent them. Before any severe damage takes place, it is necessary to ensure no drug recall takes place. The majority of drug recalls are voluntary; the manufacturer identifies an issue and recalls the affected drug. However, sometimes a drug is recalled after the FDA raises concerns.
The major difference between voluntary, requested and mandatory recalls is who initiates the process. Voluntary recalls are initiated by the manufacturer of the product and it is the manufacturer’s responsibility to contact the direct accounts about the recall. FDA-requested recalls are initiated by the FDA due to the potential for harm to those who use the product and based on an agency determination that action is necessary to protect the public health and welfare. Mandatory recalls are narrowly restricted by statute and the FDA can only order a manufacturer to recall if it fits within the parameters of the statute. Based on the gravity of the situation, the FDA will issue a public warning.
Regardless of the reason for the recall, the FDA’s role is to supervise a manufacturer’s strategy and assess and ensure the appropriateness of its handling of the recall.
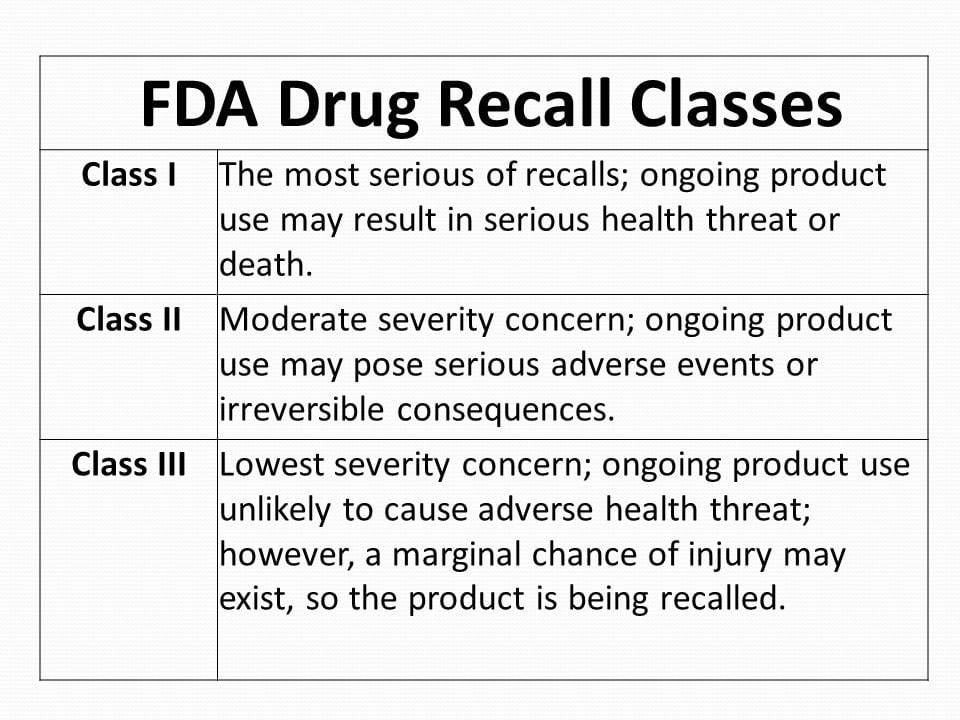
Drug Recall Class
Class 1 Recall: Reasonable probability that using the drug will cause serious adverse health consequences or death.
Class 2 Recall: Using the drug may cause temporary or medically reversible adverse health consequences, the probability of serious adverse health consequences is remote.
Class 3 Recall: Using the drug is not likely to cause adverse health consequences.
Market withdrawal: When a product has a minor violation that would not be subject to FDA legal action, a “market withdrawal” occurs. The product is removed by the firm from the market or corrects the violation.
Medical device safety alert: Released in circumstances where a medical device may present an unreasonable risk of substantial harm. These situations also are considered recalls in certain cases.
What do I do if my prescription is recalled?
If a prescription medication that you have been taking is recalled, do not panic. Stop taking the medication immediately, and call your doctor or contact a pharmacist and ask for a recommended replacement. Read the available materials from the FDA or the manufacturer to understand the reason for the recall.