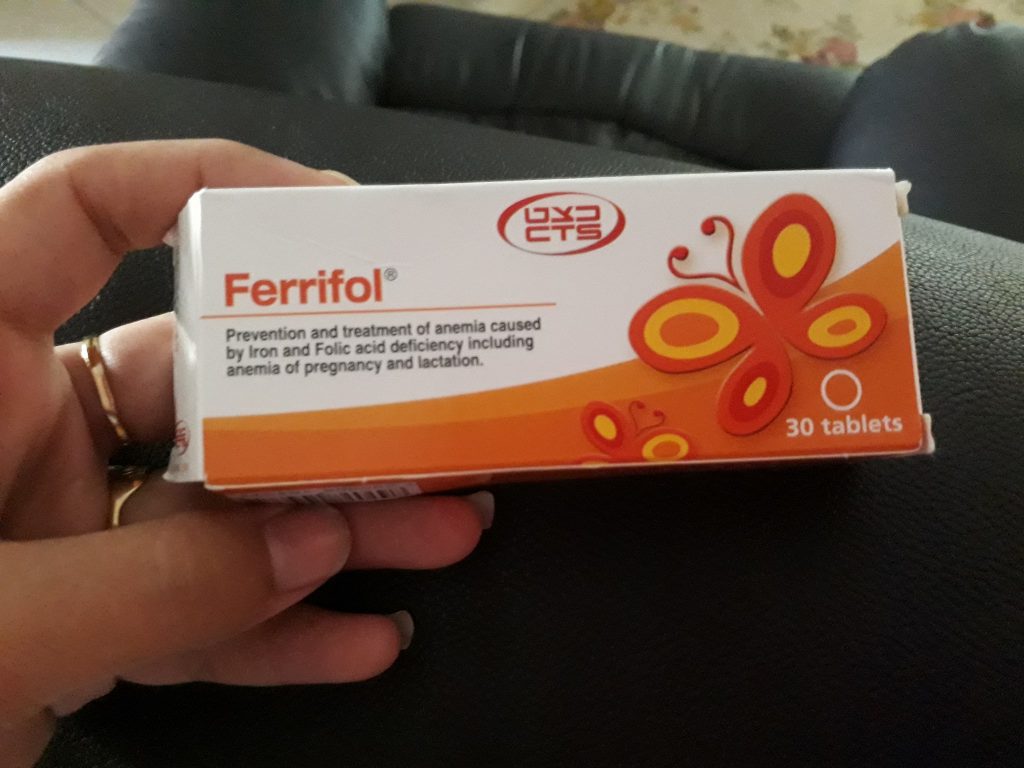
Ferrifol: Uses, Dosage, Side Effects, Interactions

Ferrifol tablet is a nutritional supplement used for the prevention and treatment of anemia due to iron deficiency and folic acid, especially during and after pregnancy and during breastfeeding.
Each tablet of Ferrifol contains Iron as Iron III Hydroxide Polymaltose Complex 100 mg and folic acid 0.4 mg as active ingredients. Iron is an essential constituent of red blood cells (hemoglobin), muscle cells (myoglobin), and iron-containing enzymes. Folic acid is a vitamin that is important for fetal development. Folic acid deficiency in the early weeks of pregnancy may lead to fetal malformations.
According to studies, iron in combination with folic acid has a beneficial impact on anemia at term and should be routinely used in pregnant women to reduce the incidence of anemia due to increased demands during pregnancy.
Before using the medicine:
Do not use this medicine if:
• You are sensitive (allergic) to the active ingredients or to any of the additional components the medicine contains.
• You have an excess of iron in your body (e.g., deficiency caused by a rare disease of iron accumulation, which may lead to a build-up of iron in the tissues).
• You have a problem with efficient utilization of iron (e.g., when anemia is caused by inefficient utilization of iron).
• You have anemia that is not caused by iron deficiency (e.g., anemia caused by increased hemoglobin breakdown or by vitamin B12 deficiency).
Special warnings regarding the use of the medicine
Before treatment with Ferrifol Tablets, inform the doctor if:
• You have an infection or a tumor.
• You have vitamin B12 deficiency. The folic acid in this medicine may mask vitamin B12 deficiency.
• You have received blood transfusions since there is a risk for an excess of iron due to receiving additional iron.
• You have other diseases or allergies.
Tests and follow-up: Before starting to use the medicine, the doctor will refer you for a blood test to check your blood iron and hemoglobin levels. If your symptoms are not caused by iron deficiency, this medicine will not be effective for you. During treatment with this medicine, the doctor will carry out periodic examinations, and may also refer you for blood tests. This referral is normal and should not concern you.
Drug-drug interactions: If you are taking or have recently taken other medicines including non-prescription medicines and dietary supplements, tell the doctor or the pharmacist. Especially if you are taking:
• Injected iron preparations – using such preparations together with this medicine is not recommended.
• Medicines for the treatment of epilepsy, especially phenytoin.
• Chloramphenicol – a medicine for the treatment of bacterial infections. The doctor will monitor you if you are taking both of these medicines together.
Use of the medicine and food: The medicine should be taken during or after a meal.
Pregnancy, breastfeeding, and fertility: No adverse effects of Ferrifol Tablets have been observed on the fetus or on women during pregnancy. It is unknown whether iron passes into breastmilk. If you are pregnant, planning to become pregnant, or breastfeeding, consult the doctor before using the medicine.
Driving and operating machinery
Ferrifol Tablets does not affect your ability to drive and or operate machinery. Important information about some ingredients of the medicine: Each Ferrifol tablet contains 1.5 mg aspartame. Aspartame is a source of phenylalanine and may cause harm if you have phenylketonuria (a rare hereditary disorder that causes accumulation of phenylalanine, since the body cannot excrete it normally).
How should you use the medicine?
Check with the doctor or pharmacist if you are uncertain about the dosage and how to use the medicine. The generally accepted dosage is:
For adults and children above 12 years of age: For prevention of anemia: one tablet per day.
For treatment of anemia: 3 tablets per day in a single daily dose or in divided doses.
Do not exceed the recommended dose. The tablet may be chewed or swallowed whole.
How long does the treatment usually last?
Depends on the extent of iron and or folic acid deficiency. If you took an overdose or by mistake a child swallowed this medicine, go immediately to the doctor or the emergency room of the hospital and take the package of the medicine with you. A too high dose of folic acid may cause changes in mental state and sleep patterns, irritability, hyperactivity, nausea, abdominal bloating, and flatulence. If you have forgotten to take this medicine at the required time, do not take a double dose.
Take the next dose at the scheduled time. Do not take medicines in the dark! Check the label and the dose every time you take the medicine. Wear glasses if you need them. If you have any other questions regarding the use of the medicine, consult the doctor or the pharmacist.
Side effects: As with any medicine, using Ferrifol Tablets may cause side effects in some users. Do not be alarmed when reading the list of side effects. You may not experience any of them. Very common side effects – side effects that occur in more than one out of ten users: Stool discoloration due to iron excretion. This phenomenon is harmless.
Common side effects – side effects that occur in 1-10 out of 100 users: Nausea, constipation, diarrhea, and abdominal pain.
Uncommon side effects – side effects that occur in 1-10 out of 1,000 users: Vomiting, teeth discoloration, gastritis, itching, rash, hives, redness in the skin, headache. Rare side effects – side effects that occur in 1-10 out of 10,000 users: Muscle cramps and pain. These side effects are usually transient. If a side effect occurs, if one of the side effects worsens, or if you suffer from a side effect not mentioned in this leaflet, consult your doctor.
Interactions
Interactions of the iron (III) hydroxide polymaltose complex (IPC) with tetracycline or aluminium hydroxide were investigated in three human studies (cross-over design, 22 patients per study). No significant reduction in the absorption of tetracycline was shown. The plasma concentration of tetracycline did not fall below the level necessary for bacteriostasis. The absorption of iron from IPC was not reduced by aluminium hydroxide and tetracycline. The iron (III) hydroxide polymaltose complex can therefore also be administered at the same time as tetracyclines or other phenolic compounds, as well as aluminium hydroxide. Studies in rats with tetracycline, aluminium hydroxide, acetylsalicylate, sulfasalazine, calcium carbonate, calcium acetate, calcium phosphate in combination with vitamin D3, bromazepam, magnesium aspartate, D-penicillinamine, methyldopa, paracetamol and auranofin have not shown any interactions with the iron (III) hydroxide polymaltose complex. There were also no interactions of the iron(III) hydroxide polymaltose complex with food components, such as phytic acid, oxalic acid, tannin, sodium alginate, choline and choline salts, vitamin A, vitamin D3 and vitamin E, soy oil and soy flour observed in in-vitro studies. These results indicate that iron (III) hydroxide polymaltose complex can be taken during or immediately after food intake. The haemoccult test (selective for Hb) for the detection of occult blood is not impaired, and therefore there is no need to interrupt the therapy. Concomitant administration of parenteral iron preparations and Ferrifol is not indicated because it would reduce the absorption of the oral iron preparation. Folic acid could increase the metabolism of phenytoin resulting in decreased concentrations of phenytoin in the serum, particularly in patients with folic acid deficiency. There may be an increased frequency of epileptic seizures in some patients. Patients who take phenytoin or another antiepileptic/anticonvulsive medicinal product should consult a doctor before taking a folic acid supplement. There are reports that the concurrent administration of chloramphenicol and folic acid in patients with folic acid deficiency may result in antagonism of the haematopoietic response to folic acid. Although the importance and mechanism of this interaction is unclear, the haematopoietic response to Page 4/8 folic acid in patients taking both medicinal products concomitantly should be carefully monitored.
How to store the medicine?
Avoid poisoning! This medicine and any other medicine must be kept in a closed place out of the reach and sight of children and/or infants to avoid poisoning. Do not induce vomiting without an explicit instruction from the doctor. Do not use the medicine after the expiry date (exp. date) appearing on the package. The expiry date refers to the last day of that month. Store at a temperature lower than 25°C, in a dark place.
Additional information: In addition to the active ingredients, the medicine also contains: Dextrates, Polyethylene Glycol 6000, Purified Talc, Aspartame, Magnesium Stearate, Chocolate Essence
What does the medicine look like and what are the contents of the package?
The package contains 20 or 30 tablets in blister packs. A brown-white speckled tablet, with a chocolate aroma. Not all pack sizes may be marketed.