FDA Grants Approval to UCB’s Bimzelx (bimekizumab-bkzx) for Moderate to Severe Plaque Psoriasis Treatment

UCB, a global biopharmaceutical company, has announced the approval of Bimzelx® (bimekizumab-bkzx) by the U.S. Food and Drug Administration (FDA) for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
This approval is significant as Bimekizumab is the first and only psoriasis treatment that selectively inhibits two key cytokines involved in the inflammatory processes – interleukin 17A (IL-17A) and interleukin 17F (IL-17F).
Key points regarding the FDA approval of Bimzelx:
1. Approval and Significance: Bimzelx (bimekizumab-bkzx) has been approved by the FDA for the treatment of moderate to severe plaque psoriasis. This approval represents a significant milestone in improving the standard of care for psoriasis treatment.
2. Efficacy and Safety: The approval is based on data from three Phase 3 clinical trials (BE READY, BE VIVID, and BE SURE) involving 1,480 adults with moderate to severe plaque psoriasis. These studies evaluated the effectiveness and safety of Bimzelx. Results demonstrated that Bimzelx achieved superior levels of skin clearance compared to other existing treatments.
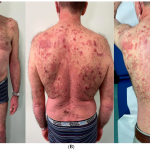
3. Psoriasis Impact: Psoriasis is a skin condition that affects over 7.5 million adults in the United States. Beyond the skin symptoms such as itching and flaking, it can have a significant impact on patients’ lives, affecting work, relationships, and overall well-being.
4. Recommended Dosage: The recommended dosage of Bimzelx for psoriasis patients is 320 mg, administered as two subcutaneous injections of 160 mg each at Weeks 0, 4, 8, 12, and 16, followed by injections every 8 weeks. For patients weighing over 120 kg, a dose of 320 mg every 4 weeks after week 16 may be considered.
5. Additional Applications: With Bimzelx approved for psoriasis, the company plans to submit applications for additional indications in the U.S., expanding its potential uses.
6. Support from National Psoriasis Foundation: The President and CEO of the National Psoriasis Foundation expressed hope that new treatments like Bimzelx will improve outcomes for patients and alleviate the physical and emotional burden of psoriasis.
7. Safety Information: The safety information for Bimzelx includes warnings about the risk of suicidal ideation and behavior, infections, tuberculosis, liver biochemical abnormalities, inflammatory bowel disease, and immunizations.
8. Most Common Adverse Reactions: The most common adverse reactions reported include upper respiratory infections, oral candidiasis, headache, injection site reactions, tinea infections, and others.
9. About Bimzelx: Bimzelx is a humanized IgG1 monoclonal antibody designed to selectively inhibit IL-17A, IL-17F, and IL-17AF cytokines, which are involved in the inflammatory processes associated with psoriasis.
10. About UCB: UCB is a global biopharmaceutical company focused on the discovery and development of innovative medicines for people living with severe diseases of the immune system or the central nervous system.
The FDA’s approval of Bimzelx offers a new and potentially more effective treatment option for individuals with moderate to severe plaque psoriasis, which can be a debilitating condition affecting millions of people. It represents a promising development in the field of dermatology and autoimmune diseases.