FDA Approves Vaporized Hydrogen Peroxide as Established Sterilization Method for Medical Devices

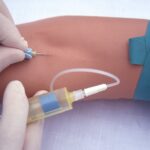
In a significant development, the U.S. Food and Drug Administration (FDA) has officially recognized vaporized hydrogen peroxide (VHP) as an established method of sterilization for medical devices. This acknowledgement comes in light of VHP’s long-standing track record of safety and efficacy. The FDA has amended its final guidance, titled “Submission and Review of Sterility Information in Premarket Notification (510(k)) Submissions for Devices Labeled as Sterile,” explicitly listing VHP as an example of an Established Category A sterilization method.
This crucial update is poised to expedite the broader adoption of VHP as a sterilization method within the medical device industry. More significantly, it aligns with the FDA’s comprehensive approach to curbing the use of ethylene oxide (EtO) wherever possible. The agency aims to bolster the medical device supply chain’s resilience while encouraging alternatives to EtO, the most commonly used sterilization method for medical devices in the United States.
Effective sterilization processes are imperative to ensure the safety of certain medical devices by neutralizing or eliminating potentially harmful microorganisms. Notably, these processes must achieve this without causing any damage to the devices themselves. Devices labeled as sterile often require premarket submissions that demonstrate the effectiveness of the sterilization process, consistent with internationally accepted consensus standards recognized by the FDA.
EtO currently accounts for over 20 billion devices sold in the U.S. annually, representing roughly 50% of devices requiring sterilization. Since 2019, the FDA has actively promoted the development of alternatives to EtO, introducing various programs and initiatives to foster innovation in medical device sterilization. This includes the establishment of Sterilization Master File Pilot programs, innovation challenges to reduce EtO emissions, and the exploration of new sterilization methods or technologies. The FDA has also engaged proactively with the industry to advance innovative alternatives to EtO.
Dr. Suzanne Schwartz, the director of the Office of Strategic Partnerships and Technology Innovation in the FDA’s Center for Devices and Radiological Health, emphasized the agency’s commitment to protecting public health. The addition of vaporized hydrogen peroxide to the Established Category A sterilization methods is seen as a strategic move to fortify the supply chain for sterilized devices, mitigating the risk of potential shortages and preventing medical device scarcity.
Methods classified as Established Category A sterilization methods, due to their long history of safe and effective use on medical devices, include moist heat, dry heat, EtO, and radiation. With the recent inclusion of VHP in this category, the FDA anticipates strengthening the industry’s ability to adopt alternative sterilization processes that pose fewer risks to the environment and surrounding communities.
The FDA remains steadfast in its commitment to minimizing adverse impacts on the environment and public health. As the field of sterilization continues to advance, the FDA pledges to explore additional modalities that offer safe and effective sterilization methods, ensuring the best protection for public health.