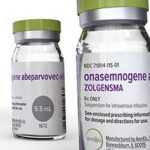
Elevidys: Uses, Benefits, Dosage, Side Effects, Price Tag

Elevidys (delandistrogene moxeparvovec-rokl) is a new gene therapy, for the treatment of Duchenne muscular dystrophy (DMD) in children. This therapy recently obtained approval from the U.S. Food and Drug Administration, making it the very first gene therapy to receive approval for addressing DMD, a debilitating genetic disorder.
Duchenne muscular dystrophy is a rare and progressive neuromuscular disease that primarily affects young boys. It is caused by a mutation in the dystrophin gene, which leads to the progressive degeneration and weakening of muscles throughout the body. This condition significantly impacts mobility, muscle strength, and overall quality of life for those affected.
Elevidys offers a promising solution by utilizing gene therapy techniques to target and address the root cause of DMD. Elevidys is an innovative gene therapy that utilizes an adeno-associated virus vector to target and treat a specific population of patients. It is specifically indicated for the treatment of ambulatory pediatric patients between the ages of 4 and 5 who have been diagnosed with Duchenne muscular dystrophy (DMD) and possess a confirmed mutation in the DMD gene.
The approval for this indication was granted under an accelerated approval pathway, which is based on the observed expression of Elevidys microdystrophin in skeletal muscle among patients who received treatment with Elevidys. However, the continued approval for this particular indication is contingent upon further verification and description of clinical benefit through confirmatory trials.
This gene therapy approach holds great potential in addressing the underlying cause of DMD by introducing the microdystrophin gene into the affected individuals. The adeno-associated virus vector acts as a delivery system, enabling the integration of the microdystrophin gene into the patients’ cells and promoting the production of functional microdystrophin protein within the skeletal muscles.
The accelerated approval of Elevidys for this specific patient population represents an important step forward in the treatment of DMD. It signifies that the therapy has shown promising early results in terms of microdystrophin expression, and further studies will be conducted to validate its clinical benefits. These confirmatory trials will provide additional evidence to support the long-term efficacy and safety of Elevidys in improving the health and functional outcomes of patients with DMD.
Dosage
Elevidys is available as a suspension intended for intravenous infusion. The nominal concentration of the suspension is 1.33 × 1013 vector genomes (vg) per milliliter (mL). This specific concentration is chosen to provide the appropriate therapeutic dose for each patient based on their body weight.
Elevidys is supplied in a customized kit that includes ten to seventy single-dose vials, each containing 10 mL of the suspension. The kit is tailored to meet the dosage requirements based on the patient’s body weight, ensuring accurate administration of the therapy.
Elevidys is administered as a single-dose intravenous infusion. Prior to treatment initiation, patients should be selected based on their anti-AAVrh74 total binding antibody titers, which should be less than 1:400.
The recommended dosage of Elevidys is 1.33 × 1014 vector genomes (vg) per kilogram of body weight. This dosage is determined based on the patient’s weight and aims to optimize the therapeutic response. It is essential to assess liver function, platelet counts, and troponin-I levels before the infusion to ensure patient safety and monitor potential side effects.
If a patient has a concurrent infection, it is advisable to postpone Elevidys treatment until the infection has been resolved to prevent any complications that may arise from administering the therapy during an active infection.
To prepare for the infusion, it is recommended to initiate a corticosteroid regimen one day before the scheduled treatment. This corticosteroid regimen typically lasts for a minimum of 60 days and may require adjustment in patients with liver function abnormalities. Corticosteroids are often prescribed alongside gene therapies to mitigate potential immune responses and optimize treatment outcomes.
Elevidys is administered as an intravenous infusion over a duration of 1-2 hours. It is important to control the infusion rate at less than 10 mL/kg/hour to ensure proper delivery and minimize the risk of adverse events associated with rapid infusion.
Contraindications
Elevidys should not be used in patients who have any deletion in exon 8 and/or exon 9 of the DMD gene. This contraindication is in place due to specific genetic factors that may impact the effectiveness or safety of the treatment in individuals with these particular gene deletions.
It is important to thoroughly evaluate the patient’s genetic profile and confirm the absence of exon 8 and exon 9 deletions before considering the administration of Elevidys. If these deletions are present, alternative treatment options should be explored in consultation with healthcare professionals familiar with the patient’s condition and medical history.
Adhering to these contraindications ensures that Elevidys is used appropriately and optimally targets the specific genetic mutations associated with Duchenne muscular dystrophy (DMD).
Precautions
Elevidys treatment requires certain precautions to ensure patient safety and optimize treatment outcomes. These precautions include:
1. Acute Serious Liver Injury: There have been reports of acute serious liver injury in patients receiving Elevidys. It is essential to monitor liver function before the infusion and continue monitoring weekly for the first three months after treatment. Monitoring should be continued until liver function results return to normal. If acute serious liver injury is suspected, it is recommended to consult with a specialist for further evaluation and management.
2. Immune-mediated Myositis: Patients with specific deletions in the DMD gene (exons 1 to 17 and/or exons 59 to 71) may be at higher risk of experiencing severe immune-mediated myositis reactions. If symptoms of myositis occur, such as unexplained increased muscle pain, tenderness, or weakness, additional immunomodulatory treatments, such as immunosuppressants (e.g., calcineurin-inhibitor) in combination with corticosteroids, should be considered. Close monitoring and prompt intervention are necessary in these cases.
3. Myocarditis: Cases of myocarditis (inflammation of the heart muscle) and elevated levels of troponin-I (a marker of heart muscle damage) have been observed in patients treated with Elevidys. Regular monitoring of troponin-I levels should be conducted before the infusion and weekly during the first month after treatment to detect any signs of myocarditis promptly.
4. Pre-existing Immunity against AAVrh74: It is important to perform baseline testing to determine the presence of anti-AAVrh74 total binding antibodies in patients before administering Elevidys. Pre-existing immunity may impact the efficacy and safety of the treatment. Test results will help inform treatment decisions and ensure appropriate management.
By adhering to these precautions, healthcare professionals can closely monitor patients receiving Elevidys, promptly address potential adverse events, and ensure the best possible treatment outcomes while prioritizing patient safety.
Side Effects
Based on studies conducted with Elevidys, the most common side effects, with an incidence of 5% or higher, are as follows:
1. Vomiting and Nausea: These gastrointestinal symptoms, including vomiting and nausea, have been reported in patients receiving Elevidys treatment.
2. Liver Function Test Increased: Elevidys treatment may lead to elevated levels of liver function markers in blood tests. Monitoring liver function is important to detect any abnormalities and ensure appropriate management.
3. Pyrexia: Pyrexia refers to an elevated body temperature or fever. This adverse reaction has been observed in patients undergoing Elevidys treatment.
4. Thrombocytopenia: Thrombocytopenia refers to a lower than normal platelet count in the blood. This adverse event has been reported in some patients receiving Elevidys.
It is crucial for healthcare providers to closely monitor patients undergoing Elevidys treatment for any adverse reactions. Prompt identification and management of these adverse events can help ensure patient safety and well-being.
Patients and caregivers should be aware of the potential adverse reactions associated with Elevidys and promptly report any concerning symptoms to their healthcare providers. Regular communication and follow-up with healthcare professionals are essential to address any adverse events and provide appropriate support throughout the treatment process.
Price
Sarepta Therapeutics Inc., the pioneering pharmaceutical company responsible for the development of Elevidys, has revealed that a one-time intravenous treatment for Duchenne muscular dystrophy (DMD) with Elevidys will cost $3.2 million. While this price may seem substantial, it is important to consider the unique nature of gene therapies and their potential to offer long-term benefits to patients.
In the United States, it is customary for the majority of medication costs to be covered by insurance providers. Given the significant impact of DMD on patients’ lives and the potential for Elevidys to address the underlying cause of the disease, insurance coverage plays a crucial role in ensuring access to this innovative therapy. Insurers typically evaluate the clinical benefits and cost-effectiveness of treatments before making coverage decisions.
The pricing of gene therapies reflects the substantial investment required for research, development, and manufacturing processes involved in creating these advanced treatments. It also takes into account the relatively limited patient population that can benefit from such therapies, which affects economies of scale.
As with any novel therapy, ongoing discussions and collaborations among healthcare providers, patients, insurers, and pharmaceutical companies are essential to striking a balance between innovation, affordability, and accessibility. This enables patients with DMD to receive the much-needed treatment they deserve and lays the foundation for future advancements in gene therapies and rare disease treatments.