Do Not Use Cardinal Health Monoject Syringes with Syringe Pumps and PCA Pumps
The U.S. Food and Drug Administration (FDA) is alerting health care providers and facilities not to use Cardinal Health Monoject syringes with syringe pumps and patient-controlled analgesia (PCA) pumps while the FDA further evaluates this issue.
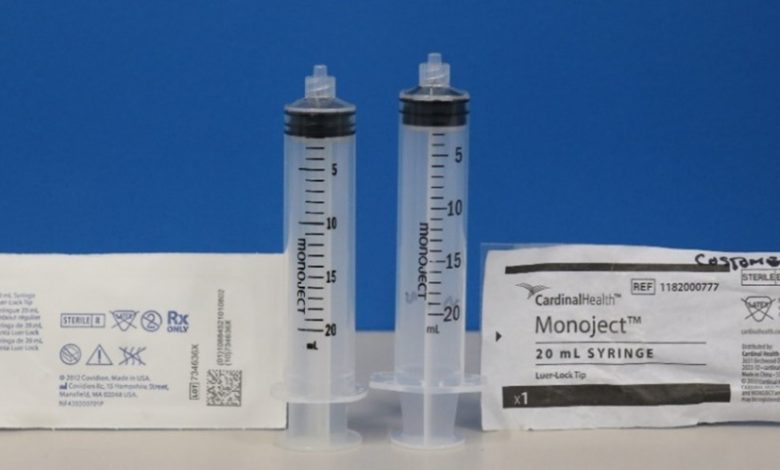
In June 2023, Cardinal Health began distributing Monoject syringes branded as “Cardinal Health” Monoject syringes. These new syringes differ from the previously branded “Covidien” Monoject syringes as they have different dimensions (example shown in image below) and are made by a different contract manufacturer. The dimensional changes made to the Cardinal Health Monoject syringes, when used with syringe pumps or PCA pumps, may result in recognition, compatibility, and pump performance issues such as overdose, underdose, delay in therapy, and delays in occlusion alarms.
On September 20, 2023, Cardinal Health initiated a recall for certain Cardinal Health Monoject single use Luer-lock tip syringes to not be used with syringe pumps. This recall is a correction, not a product removal. The FDA classified this as a Class I recall, the most serious type of recall. However, the FDA has concerns that the information provided by Cardinal Health has not sufficiently mitigated the risk of incompatibility when Cardinal Health Monoject syringes are used with other pumps, specifically PCA pumps. In addition, the FDA has concerns that the risk of incompatibility could apply to additional sizes of Cardinal Health Monoject syringes. The FDA is working with the manufacturer to address these questions.
Recommendations for Health Care Providers
At this time, the FDA recommends that health care providers and facilities:
- Do not use Cardinal Health Monoject syringes with syringe pumps or PCA pumps.
- You may continue to use Covidien Monoject syringes with syringe pumps or PCA pumps.
- Be aware that both brands of syringes state only “monoject” on the syringe itself and do not include the company name (see example in the image above).
- Keep the outer packaging with the Covidien Monoject syringe prior to using it with a syringe pump or PCA pump in order for end-users to verify that the syringe can be used.
- Stay alert for further updates and recommendations from the FDA or manufacturers.
- Report any problems with Cardinal Health Monoject syringes to the FDA.
Background
Cardinal Health Monoject disposable syringes are used to inject fluid into or withdraw fluids from the body. When used with syringe pumps and PCA pumps, disposable syringes are loaded with fluid or medications and placed into the pump.
Syringe pumps deliver solutions such as fluids, medications, and blood products to patients. PCA pumps typically deliver pain medications.
Currently, the FDA has received over a dozen Medical Device Reports (MDRs) involving delay in therapy as well as inaccurate therapy (overdose or underdose) associated with the use of Cardinal Health Monoject syringes with a syringe pump or a PCA pump. Currently, the FDA is not aware of any deaths associated with this issue.
FDA Actions
The FDA will continue to:
- Work with Cardinal Health and other applicable manufacturers to further evaluate the issue and identify additional mitigation strategies as needed.
- Monitor reports of adverse events related to the issue.
- Keep health care providers and the public informed if significant, new information or recommendations become available.
Other Affected Devices
The following list of Medical Device Recall Database entries are from infusion pump manufacturers that use Cardinal Health Monoject syringes. The Unique Device Identifier (UDI) information provided by the manufacturer can be found in the “Code Information” section of each entry. The FDA continues to work with syringe pump and PCA pump manufacturers, and this list of recalls associated with Cardinal Health Monoject syringes will be updated as additional recalls are initiated.
| Manufacturer Name | Product Name | Version or Model | Date Recall Initiated by the Manufacturer |
| Carefusion 303, Inc. | Alaris BD Alaris BD Alaris | Alaris PCA Module 8120 BD Alaris PCU REF 8015 BD Alaris Syringe Module, REF 8110 | September 15, 2023 |
Reporting Problems to the FDA
The FDA encourages health care providers to report any adverse events or suspected adverse events experienced with the Cardinal Health Monoject syringes, or any medical device.
- Health care personnel employed by facilities that are subject to the FDA’s user facility reporting requirements should follow the reporting procedures established by their facilities.
- Voluntary reports can be submitted through MedWatch, the FDA Safety Information and Adverse Event Reporting program.
- Device manufacturers and user facilities must comply with the applicable Medical Device Reporting (MDR) regulations.
By promptly reporting adverse events, you can help the FDA identify and better understand the risks associated with medical devices.
Contact Information
If you have questions about this letter, contact the Division of Industry and Consumer Education (DICE).