Cardinal Health Issues Recall for Urology and Operating Room Kits Due to Sterility Concerns
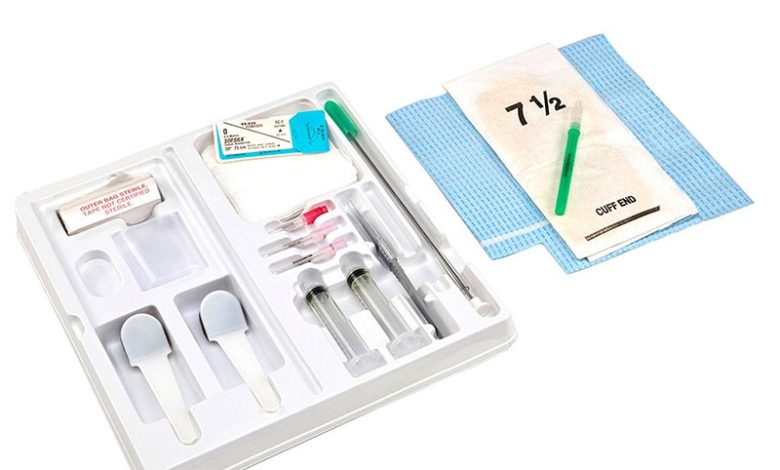
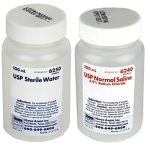
In response to the voluntary product recall initiated by Nurse Assist, LLC, Cardinal Health has issued a medical device recall on December 5, 2023. The recall specifically targets Covidien and Cardinal Health™ brand urology and operating room-specific kits and trays containing 0.9% sodium chloride irrigation USP and sterile water for irrigation USP supplied by Nurse Assist. The recall is prompted by concerns regarding sterility assurance, posing a potential risk of non-sterile product distribution.
Risk to Vulnerable Populations
Patients who are immunocompromised, particularly those in high-risk populations, face the possibility of severe or life-threatening adverse events if exposed to the affected product. As of now, there have been no reported cases of harm to patients. Cardinal Health emphasizes that patient safety is their utmost priority.
Identified Affected Codes and Lot Numbers
Following a comprehensive review of the Nurse Assist recall’s impact, Cardinal Health has identified specific Covidien and Cardinal Health™ brand codes and lot numbers that are affected by the recall.
[Link to Affected Covidien and Cardinal Health™ brand codes and lot numbers]
Instructions for Customers
Customers who received kits or trays with the listed affected lots are directed to take the following actions:
- QUARANTINE: Isolate the affected kits/trays.
- ALERT: Inform clinicians about the recalled component(s).
- AFFIX WARNING LABEL: Attach a prominent warning label to the front of each kit/tray, clearly instructing clinicians to remove and discard the recalled component(s).
- NOTIFY: Reach out to other departments, facilities, or customers within the hospital system if affected kits/trays have been transferred. Provide a copy of the notice and recall acknowledgement form to them.
- RETURN ACKNOWLEDGMENT FORM: Complete and return the enclosed Acknowledgment Form confirming the receipt of the recall notice and the disposal of over-labeled products via fax to 614.652.9648.
Contact Information for Inquiries
For additional information or questions related to this recall, customers are urged to contact Cardinal Health via email at fieldcorrectiveaction@cardinalhealth.com or by calling 800-292-9332.
Cardinal Health assures that they are committed to addressing this issue promptly to ensure the safety of patients and healthcare providers. Regular updates will be provided as the situation evolves.