How Sertraline Treatment Affects The Eye
Sertraline is a widely used antidepressant medication that belongs to the class of selective serotonin reuptake inhibitors (SSRIs). It works by increasing the levels of serotonin in the brain, which is a neurotransmitter that helps regulate mood, emotions, and behavior. While sertraline is generally well-tolerated, like any medication, it can cause side effects.
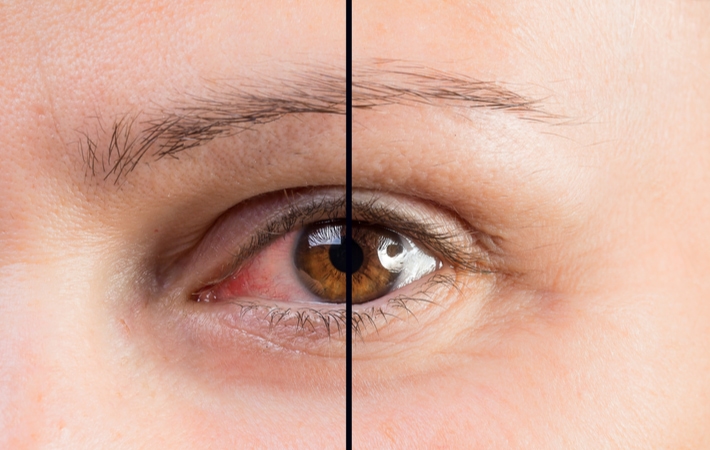
One of the possible side effects of sertraline is changes in vision, particularly in the eye. In this article, we will discuss how sertraline treatment affects the eye in detail.
Sertraline and Blurred Vision
Blurred vision is one of the most common side effects of sertraline. It can occur at any time during treatment, but it is most likely to occur during the first few weeks of treatment. Blurred vision is usually mild and transient, and it typically resolves on its own within a few days to weeks without any treatment. However, in some cases, it may persist or worsen over time, which may require medical attention.
The exact mechanism of how sertraline causes blurred vision is not well understood. However, it is thought to be related to the medication’s effect on the autonomic nervous system, which regulates the pupils’ size and the muscles that control the lens’s shape. Sertraline can cause the pupils to dilate or constrict, which can lead to blurred vision or difficulty focusing.
Sertraline and Dry Eyes
Dry eyes are another common side effect of sertraline. It occurs when the eyes do not produce enough tears or when the tears evaporate too quickly. Dry eyes can cause discomfort, itching, burning, and redness. It can also affect vision, causing blurred or fluctuating vision.
The mechanism of how sertraline causes dry eyes is also not well understood. However, it is thought to be related to the medication’s effect on the autonomic nervous system, which regulates tear production. Sertraline can reduce the amount of tears produced by the eyes, which can lead to dry eyes.
Sertraline and Eye Pain
Eye pain is another reported side effect of sertraline. It is usually mild and transient, and it typically resolves on its own within a few days to weeks without any treatment. However, in some cases, it may persist or worsen over time, which may require medical attention.
The mechanism of how sertraline causes eye pain is not well understood. However, it is thought to be related to the medication’s effect on the autonomic nervous system, which regulates the muscles that control the eye’s movement. Sertraline can cause the muscles to contract or relax, which can lead to eye pain or discomfort.
Sertraline and Sensitivity to Light
Sensitivity to light, also known as photophobia, is another reported side effect of sertraline. It occurs when the eyes become overly sensitive to light, causing discomfort or pain. Sensitivity to light can cause headaches, eye strain, and difficulty reading or working on a computer.
The mechanism of how sertraline causes sensitivity to light is not well understood. However, it is thought to be related to the medication’s effect on the autonomic nervous system, which regulates the muscles that control the pupil’s size. Sertraline can cause the pupils to constrict or dilate, which can lead to sensitivity to light.
Sertraline and Changes in Color Vision
Changes in color vision, such as seeing everything in shades of yellow or green, have also been reported in people taking sertraline. These changes are usually mild and transient, and they typically resolve on their own within a few days to weeks without any treatment. However, in some cases, they may persist or worsen over time, which may require medical attention.
The mechanism of how sertraline causes changes in color vision is not well understood. However, it is thought to be related to the medication’s effect on the visual pathways in the brain. Serotonin, the neurotransmitter that sertraline increases, has been shown to affect color vision in animal studies. It is possible that sertraline may also affect color vision in humans by altering the levels of serotonin in the brain.
Sertraline and Eye Twitching
Eye twitching, also known as eyelid myokymia, is a rare side effect of sertraline. It occurs when the muscles around the eye contract and relax involuntarily, causing the eyelid to twitch or flutter. Eye twitching is usually mild and transient, and it typically resolves on its own within a few days to weeks without any treatment. However, in some cases, it may persist or worsen over time, which may require medical attention.
The mechanism of how sertraline causes eye twitching is not well understood. However, it is thought to be related to the medication’s effect on the nervous system. Sertraline can affect the levels of neurotransmitters in the brain, which can alter the signals that control muscle movements, leading to eye twitching.
Sertraline and Angle-Closure Glaucoma
Angle-closure glaucoma is a rare but serious side effect of sertraline. It occurs when the fluid inside the eye cannot drain properly, leading to a sudden increase in eye pressure. This increase in pressure can cause damage to the optic nerve and lead to vision loss if left untreated. Symptoms of angle-closure glaucoma include eye pain, redness, blurred vision, and seeing halos around lights.
The exact mechanism of how sertraline causes angle-closure glaucoma is not well understood. However, it is thought to be related to the medication’s effect on the nervous system. Sertraline can affect the levels of neurotransmitters in the brain, which can alter the signals that control the muscles that regulate the flow of fluid in the eye.
Conclusion
Sertraline is a commonly prescribed antidepressant medication that can cause side effects in some individuals, including changes in vision. These changes may include blurred vision, dry eyes, eye pain, sensitivity to light, changes in color vision, eye twitching, and in rare cases, angle-closure glaucoma. If you experience any changes in vision while taking sertraline, it is important to talk to your healthcare provider right away. Your doctor may recommend that you see an eye specialist to determine the cause of your symptoms and develop an appropriate treatment plan.