Ycanth Topical: Benefits, Uses, Dosage, Side Effects, Interactions
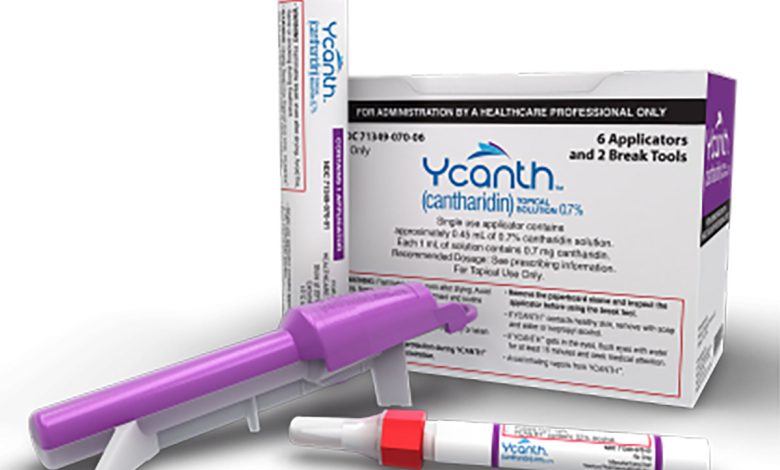
Ycanth is a specialized drug-device combination product that contains cantharidin, delivered through a single-use applicator and formulated under Good Manufacturing Practices (GMP) standards at a concentration of 0.7% w/v. As the first and only treatment approved by the U.S. Food and Drug Administration (FDA) for molluscum contagiosum, a primarily pediatric disease, Ycanth is a significant advancement in healthcare.
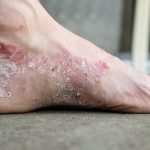
Molluscum contagiosum, primarily affecting children, is a highly contagious skin condition commonly transmitted in various settings such as households, schools, swimming pools, and other extra-curricular environments. The ease of transmission is attributed to its spread through skin-to-skin contact and the sharing of contaminated objects with its viral lesions. Therefore, to effectively prevent further transmission, topical treatment with precise administration is crucial in containing the spread of this contagious infection.
The approval of Ycanth is based on solid evidence from well-designed and controlled trials involving pediatric patients aged 2 years and older. However, it’s worth noting that the safety and efficacy of Ycanth for pediatric patients below the age of 2 years have not been established, underscoring the importance of cautious usage and adherence to the approved indications.
Due to the specialized nature of the treatment and the potential risks associated with its application, Ycanths should only be administered by healthcare professionals who have received proper training. As a result, it is not intended for home use, emphasizing the need for expert guidance and supervision during treatment.
The indication for Ycanth is clear and specific, as it is meant for the topical treatment of molluscum contagiosum in both adult and pediatric patients aged 2 years and older. This provides a much-needed treatment option for patients of various ages who are affected by this contagious skin condition.
Benefits
Ycanth offers several benefits as the first and only FDA-approved treatment for molluscum contagiosum:
1. Effective Treatment: Ycanth has been proven effective in well-designed and controlled trials involving pediatric patients aged 2 years and older. Its approval is based on solid evidence, providing confidence in its efficacy for treating molluscum contagiosum.
2. Specialized Formulation: Ycanth is formulated under Good Manufacturing Practices (GMP) standards at a concentration of 0.7% w/v, ensuring consistency and quality in each application.
3. Targeted Application: The single-use applicator allows for precise administration of the treatment. This is crucial in containing the spread of the highly contagious infection, which can be transmitted through skin-to-skin contact and the sharing of contaminated objects.
4. Pediatric Focus: Molluscum contagiosum primarily affects children, and Ycanth addresses this pediatric disease, offering a treatment option specifically tailored for younger patients.
5. Reduced Transmission Risk: By effectively treating and resolving molluscum contagiosum lesions, Ycanth helps prevent further transmission of the infection in settings such as households, schools, and other communal environments.
6. Expert Administration: Ycanth is intended for use by healthcare professionals who have received proper training. This ensures that the treatment is administered safely and effectively, minimizing potential risks associated with its application.
7. Alternative to Invasive Procedures: Before the approval of Ycanth, treatment options for molluscum contagiosum were often invasive or required painful procedures. Ycanth offers a topical, non-invasive alternative for patients.
How to use Ycanth
Ycanth is administered as a topical treatment on the skin and should only be applied by a trained healthcare provider to the areas affected by molluscum bumps. It is essential to avoid using Ycanth in the mouth, nose, genital areas, or eyes.
Multiple treatments with Ycanth may be necessary, and your healthcare provider can apply it every 3 weeks as required.
To prepare for the treatment with Ycanth:
• On the day of the procedure, do not use any topical steroids, creams, lotions, or sunscreen on the skin areas with molluscum bumps.
• Your healthcare provider, who has received specific training in the application of Ycanth, will wear gloves and personal protective equipment, including eye protection while applying the product to your skin.
Once Ycanth has been applied to your skin by your healthcare provider:
• Allow it to dry completely before leaving the office or clinic, which may take up to 5 minutes.
• Refrain from touching the treated areas for 24 hours or until washing, and avoid contact with your mouth, nose, genital areas, or eyes during this time as well.
• If children are being treated, ensure they do not lick or bite the treated areas, as Ycanth contains a bittering agent to deter them from ingesting it.
• The Ycanth solution contains a violet-colored dye, which may temporarily stain the treated skin areas.
If you experience discomfort or pain following the treatment, you may take over-the-counter pain relief medication as needed.
Twenty-four hours after the treatment, you can wash the treated skin area with soap and water unless your healthcare provider advises otherwise. If you develop severe blistering, pain, or other intense reactions, wash off Ycanth gently from your skin earlier. Avoid using a washcloth, abrasive material, or vigorous rubbing during this process to prevent additional discomfort.
Warnings and Precautions
• Ycanth is for topical use only. It must not be administered orally, mucosally, or into the eyes. Life-threatening or fatal toxicities can occur if ingested orally. After treatment, avoid any contact with the treatment area, including oral contact, to prevent adverse reactions.
• Ocular toxicity can occur if Ycanth comes into contact with the eyes. In case of accidental contact, immediately flush the eyes with water for at least 15 minutes.
• Local skin reactions may occur at the application site, including vesiculation, pruritus (itchiness), pain, discoloration, and erythema (redness). Avoid applying Ycanth near the eyes, mucosal tissue, or healthy skin. If Ycanth contacts unintended surfaces or healthy skin, remove it promptly. In case of severe local skin reactions, remove the application prior to the recommended 24-hour duration after treatment.
• Ycanth is flammable, even after drying. Take precautions to avoid fire, flame, or smoking near the treated lesion(s) during and after application until it is removed.
Side Effects
The most common (incidence ≥1%) reactions observed at the application site include:
• Vesiculation (formation of blisters)
• Pain
• Pruritus (itchiness)
• Scabbing
• Erythema (redness)
• Discoloration
• Application site dryness
• Edema (swelling)
• Erosion
During clinical trials, 97% of subjects treated with Ycanth experienced local skin reactions at the application site. These reactions are expected and related to the anticipated blistering response of the skin to cantharidin.
Drug Interactions
No studies evaluating the drug interaction potential of cantharidin have been conducted.
Use In Specific Populations
• Pregnancy: There are no available data on the use of Ycanth in pregnant women to assess the risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Since systemic exposure to cantharidin after topical administration is low, maternal use is not expected to result in fetal exposure to the drug.
• Lactation: Avoid applying Ycanth topical solution to areas with an increased risk of ingestion by or ocular exposure to the breastfeeding child.
Overdosage
Oral ingestion of cantharidin can lead to severe consequences, including renal failure, blistering, damage to the gastrointestinal tract, coagulopathy, seizures, and flaccid paralysis.
Storage
To maintain the stability and efficacy of Ycanth, proper storage conditions are essential. Follow the guidelines below for storing the product:
- Temperature: Store Ycanth at room temperature between 20°C to 25°C (68°F to 77°F). Avoid exposure to extreme heat or cold. Excursions from the recommended storage temperature are permitted within the range of 15°C to 30°C (59°F to 86°F). Avoid leaving the product in direct sunlight or in areas where temperatures exceed the specified range.
- Protect from Light: Ensure that Ycanth is kept in its original packaging, and protect it from exposure to light. Light-sensitive medications, like Ycanth, can degrade when exposed to excessive light. Keeping it in a dark, cool place will help preserve its effectiveness.
It is crucial to follow the storage instructions diligently to maintain the integrity of the product and ensure its maximum efficacy when applied by a trained healthcare provider. Proper storage will help guarantee that Ycanth remains safe and effective for use in treating molluscum contagiosum in patients aged 2 years and older, offering them a well-controlled and reliable treatment option.