Xdemvy: Uses, Benefits, Dosage, Side Effects, Interactions
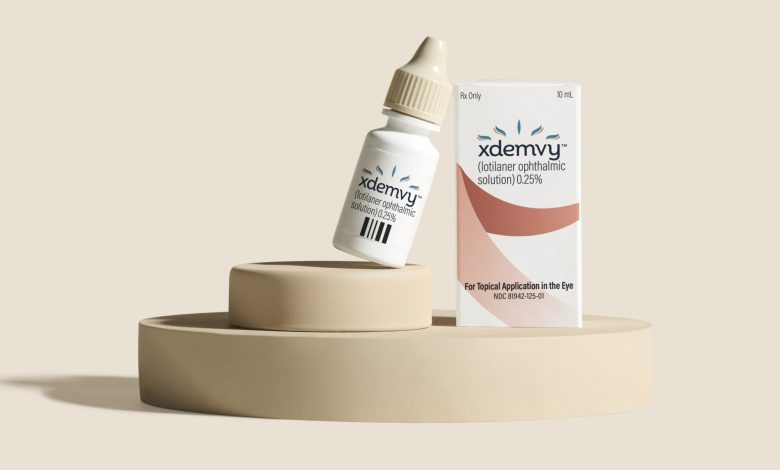
Xdemvy (lotilaner 0.25% ophthalmic) is a specialized eye drop solution with anti-parasitic properties, designed to effectively treat Demodex blepharitis in adults aged 18 years and older.
Demodex blepharitis is a condition resulting from the infestation of Demodex mites, the most common ectoparasites found in humans, in the eyelashes and eyelash follicles. It accounts for about 45% of all blepharitis cases and is particularly prevalent, reaching 84%, in individuals aged 60 and older. Common symptoms include inflammation along the eyelid margins, redness, ocular irritation, and sometimes co-occurrence with conditions like rosacea or diabetes.
The mechanism of action for Xdemvy involves inhibiting gamma-aminobutyric acid (GABA)-gated chloride channels specifically in the Demodex mites. This action leads to paralysis and eventual demise of the mite, offering relief from eyelid redness and crusting. Importantly, it’s worth noting that lotilaner, the active ingredient in Xdemvy, does not have an impact on GABA-mediated chloride channels in humans, even at doses 1100 times higher than the recommended human dose.
Xdemvy Benefits
Xdemvy offers several benefits in managing Demodex Blepharitis:
1. Treatment of Demodex Blepharitis: Xdemvy is specifically formulated to target Demodex mites that infest the eyelids. By inhibiting the gamma-aminobutyric acid (GABA)-gated chloride channels in these mites, the medication causes paralysis and death of the mites, leading to relief from symptoms associated with Demodex blepharitis.
2. Alleviation of Symptoms: Demodex blepharitis can cause various uncomfortable symptoms such as eyelid margin inflammation, redness, ocular irritation, and crusting. Xdemvy helps in reducing these symptoms, providing relief to affected individuals.
3. Limited Systemic Absorption: Xdemvy is an ophthalmic solution, meaning it is applied directly to the eyes. Due to its localized application, systemic absorption of the medication is low, reducing the risk of systemic side effects.
4. FDA Approval: Xdemvy has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of Demodex blepharitis, indicating its safety and efficacy for this specific use.
As with any medication, it’s essential to use Xdemvy as directed by your healthcare provider and to discuss any potential side effects or concerns with them. Xdemvy can provide significant benefits in managing Demodex blepharitis and improving the overall comfort and health of the eyes for affected individuals.
Dosage
Xdemvy is administered by instilling one drop in each eye twice daily, with approximately 12 hours between doses. The treatment duration typically lasts for 6 weeks. However, it’s crucial to follow the specific instructions given by a healthcare professional or as stated on the medication’s packaging.
When applying the eye drops, be sure not to touch the tip with your fingers or let it come into contact with your eyes, eyelids, face, or any other surface. This precaution is essential to reduce the risk of contamination of the solution. Using contaminated eye drop solutions can lead to severe eye damage and potential loss of vision. Therefore, it is crucial to handle the medication with care and follow proper administration instructions to ensure your safety and the effectiveness of the treatment.
The safety and effectiveness of Xdemvy in children and young adults under the age of 18 have not been established, which means it is not recommended for use in this age group until further studies are conducted and safety is confirmed.
Regarding the use of Xdemvy in pregnant women, there is limited data available, and the effects on human pregnancies are not yet known. However, animal reproduction studies with lotilaner, the active ingredient in Xdemvy, did not report any malformations. Nevertheless, the use of Xdemvy during pregnancy should only be considered if the potential benefits outweigh the potential risks, and it should be administered under the guidance of a healthcare professional.
For breastfeeding women, there is currently no data on the presence of lotilaner in human milk, its effects on breastfed infants, or its impact on milk production. However, it’s worth noting that systemic exposure to lotilaner from ocular administration is low. As with pregnancy, the decision to use Xdemvy while breastfeeding should be made with the help of a healthcare professional, weighing the potential benefits against any possible risks to the nursing infant.
Always consult a qualified healthcare professional before using any medication, especially if you have specific medical conditions or if you are pregnant or breastfeeding. They will be able to provide personalized advice based on your individual health circumstances.
Side Effects
Xdemvy may cause certain side effects, with the most common being instillation site stinging and burning, reported in approximately 10% of people using the medication.
Other side effects have been reported in less than 2% of patients and include:
1. Chalazions: These are small bumps that form on the eyelid due to a blockage of a tiny oil gland, also known as meibomian cysts or eyelid cysts.
2. Punctate Keratitis: This refers to the presence of fluffy or snowflake-like white patches on the cornea, also known as opacities of the superficial corneal stroma.
If you experience any of these side effects or any other unusual reactions while using Xdemvy, it is advisable to seek your doctor’s advice regarding the continued use of the medication.
Additionally, some precautions should be taken when using Xdemvy:
1. Eye Infection or Trauma: If you have an eye infection or experience any trauma to the eye, consult your doctor before using Xdemvy.
2. Eye Surgery: If you are scheduled for eye surgery, it’s important to inform your healthcare provider about your use of Xdemvy.
3. Conjunctivitis or Eyelid Reactions: If you develop conjunctivitis (pink eye) or any adverse reactions involving the eyelids while using Xdemvy, seek medical advice.
Use with Contact Lenses: Xdemvy contains potassium sorbate, which may cause discoloration of soft contact lenses. To prevent this, remove your contact lenses before instilling Xdemvy into your eyes. You can reinsert your lenses 15 minutes after administering the eye drops.
Use with Other Eye Drops or Ointments: Wait for at least 5 minutes after using Xdemvy before instilling any other eye drops or eye ointments in your eyes.
Always follow the recommended usage instructions and any advice provided by your healthcare professional to ensure safe and effective use of Xdemvy and to minimize the risk of side effects.
Interactions
Drug interactions with Xdemvy are not well-studied, but based on the current information available, it is not expected to interact with other medications that are taken orally (systemically) as it is not metabolized by CYP enzymes. However, since there are no extensive studies on drug interactions, caution is still advised, and it’s essential to inform your healthcare provider about all the medications you are taking, including prescription drugs, over-the-counter medicines, vitamins, and herbal supplements.
To avoid potential interactions and ensure proper effectiveness, it is recommended to wait for at least 5 minutes after administering Xdemvy before using any other eye drops or eye ointments in the same eye.
Keep in mind that the list of possible drug interactions provided here may not cover all potential interactions. Therefore, it is crucial to discuss all your medications with your healthcare provider to ensure the safe and effective use of Xdemvy.
Storage
To ensure the stability and effectiveness of Xdemvy, it should be stored within the temperature range of 15°C to 25°C (59°F to 77°F). This means keeping the medication at room temperature and avoiding exposure to extreme heat or cold.
Once the bottle of Xdemvy is opened, it can be used until the expiration date specified on the bottle. It’s crucial to follow the expiration date to ensure the medication’s potency and safety. Using the eye drops beyond the expiration date can result in reduced effectiveness and potential risks.
Always check the product packaging for specific storage instructions and expiration dates, and if you have any doubts or concerns about the medication’s condition, consult your healthcare provider or pharmacist for guidance. Proper storage and adherence to expiration dates are important for maintaining the quality of the medication and ensuring the best possible treatment outcomes.