World’s First Subcutaneous Cancer Injection Launched by NHS England

In a groundbreaking development, NHS England has unveiled a revolutionary approach to cancer treatment, offering hope to hundreds of cancer patients across the nation. This pioneering initiative involves the introduction of a subcutaneous cancer injection, heralded as a “world first,” capable of dramatically reducing treatment time by up to 75%.
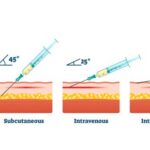
Currently, patients undergoing treatment with the life-extending anti-PD-(L)1 immunotherapy drug known as atezolizumab, produced by Roche under the brand name Tencentriq, receive it intravenously in a hospital setting. This intravenous administration typically consumes approximately 30 minutes, and for some patients facing challenges with venous access, it can extend to a daunting hour.
The pivotal moment in this healthcare breakthrough occurred with the approval of the new subcutaneous formulation by the Medicines and Healthcare products Regulatory Agency (MHRA). This approval paves the way for the wider availability of this innovative treatment option. NHS England is poised to swiftly transition eligible patients currently receiving atezolizumab to this swifter and more comfortable subcutaneous injection, promising an improved experience for hundreds of individuals.
Professor Peter Johnson, NHS England’s national director for cancer, enthusiastically welcomed this development, emphasizing that the introduction of this pioneering treatment would not only reduce hospital time for patients but also free up valuable resources in NHS chemotherapy units. This change is set to be a game-changer in the field of cancer care.
Atezolizumab, a formidable weapon in the fight against cancer, is instrumental in treating a range of malignancies, including those affecting the lung, breast, liver, and bladder.
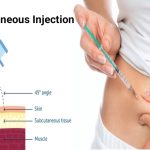
The subcutaneous version of this groundbreaking drug, known as Tencentriq SC, is formulated in collaboration with Halozyme’s recombinant human hyaluronidase enzyme, rHuPH20. Harnessing the power of ENHANZE drug delivery technology, this subcutaneous treatment takes a mere 7 minutes, making it significantly faster, more convenient, and less painful than its intravenous counterpart.
The decision to approve this innovative approach was based on robust clinical data from the Phase IB/III IMscin001 study, revealing that the levels of Tecentriq in the blood remained consistent when administered subcutaneously. Moreover, it demonstrated a safety and efficacy profile in line with the intravenous formulation. Notably, most cancer patients expressed a preference for subcutaneous delivery over intravenous infusion due to its time efficiency, ease of administration, and reduced discomfort.
Dr. Alexander Martin, a consultant oncologist at West Suffolk NHS Foundation Trust, hailed the MHRA’s announcement as “great news” for both patients and healthcare professionals. He stressed that this approval not only facilitates faster and more convenient care for patients but also empowers healthcare teams to attend to a greater number of patients throughout the day.
NHS England envisions that a significant proportion of the approximately 3600 patients starting atezolizumab treatment annually in England will opt for the time-saving injection. However, it’s worth noting that patients receiving intravenous chemotherapy in combination with atezolizumab may continue to receive the drug intravenously. Importantly, this groundbreaking advancement incurs no additional cost to the NHS, offering a beacon of hope for cancer patients and healthcare providers alike.