WHO Blames Rise in Reports of Fake Weight-Loss Drugs To Shortage of The Real Thing

In a recent statement, the World Health Organization (WHO) raised alarm about a surge in reports of counterfeit weight-loss drugs linked to the scarcity of legitimate medications, primarily those designed for diabetes management and weight loss. The global shortage, particularly in 2023, of renowned diabetes medications, such as Novo Nordisk’s Ozempic, has created a breeding ground for the production and distribution of fake drugs, posing serious health risks.
These counterfeit drugs, belonging to a category known as GLP-1 agonists, are frequently disseminated through unregulated channels, including social media platforms, contributing to a growing market for illicit pharmaceuticals. The WHO emphasized the inherent dangers associated with these fake medications, stating that they may lack efficacy, cause toxic reactions, and be produced in unhygienic conditions by unqualified personnel, potentially contaminated with harmful bacteria.
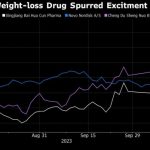
The demand for weight-loss drugs like Ozempic, Zepbound, Mounjaro, and Wegovy has surged globally, leading to shortages and creating opportunities for counterfeiters. Disturbingly, several cases have already emerged where individuals experienced dangerously low blood sugar levels after consuming suspected fake versions of Ozempic, raising concerns about the authenticity and safety of these counterfeit medications.
GLP-1 agonists, initially developed to manage type 2 diabetes, have gained popularity for their additional effect of reducing food cravings and slowing down stomach emptying, leading to significant weight loss in clinical trials—approximately 15% to 20% of body weight.
The shortage of these crucial medications in 2023, particularly in the United States, is expected to persist as pharmaceutical companies like Novo Nordisk and Eli Lilly struggle to expand their manufacturing capacities to meet the growing demand. Lilly’s CEO, David Ricks, has expressed concerns about the insufficient supply of Zepbound to meet the increasing demand, while Novo Nordisk anticipates constraints on Wegovy supplies well into 2024.
The U.S. Food and Drug Administration (FDA) has acknowledged shortages of Wegovy and one dosage of Lilly’s diabetes drug Mounjaro, further highlighting the severity of the situation. The WHO cautioned that prolonged shortages, coupled with the proliferation of counterfeit drugs, would disproportionately affect patients with type 2 diabetes.
To mitigate the risks associated with fake medications, the WHO advised consumers to procure their medicines from authorized and regulated suppliers, emphasizing the importance of healthcare professionals adhering to good prescribing and distribution practices. As the pharmaceutical industry grapples with increasing demand, safeguarding the integrity of these medications becomes imperative to ensure the well-being of patients worldwide.