MiraLAX for Kids: Safety, Dosage, Side Effects, Alternatives

According to Mayo Clinic, constipation in children is a common problem. A constipated child has infrequent bowel movements or hard, dry stools. Normally, as food moves through the colon, the colon absorbs water while it makes stool. Muscle movements (contractions) push the stool toward the rectum. When the stool gets to the rectum, most of the water has been soaked up. The stool is now solid.
If your child has constipation, the colon’s muscle movements are too slow. This makes the stool move through the colon too slowly. The colon absorbs too much water. The stool gets very hard and dry.
Once a child becomes constipated, the problem can quickly get worse. Hard, dry stools can be painful to push out. So the child may stop using the bathroom because it hurts. Over time, the colon will not be able to sense that stool is there. Up to about 30% of kids experience constipation. And when kids are constipated, they can get irritable, frustrated, and generally unhappy.
What is MiraLAX?
MiraLAX is an over-the-counter osmotic laxative containing Polyethylene Glycol 3350 also known as PEG 3350. It is used to treat constipation. MiraLAX comes as a flavorless powder that you mix with four to eight ounces of water, juice, or other liquid. The powder comes in bottles or single-serve packets. It’s typically used for short-term treatment, but in some cases, it’s used long-term to treat chronic (long-lasting) constipation. MiraLAX is also sometimes used for colonoscopy bowel preparation.
Generally, taking MiraLAX will cause a bowel movement within one to three days of taking it. One study of its effectiveness focused on people who had fewer than two bowel movements per week. MiraLAX increased their number of bowel movements to 4.5 per week, compared to 2.7 per week in people taking a placebo. Another study found that 52 percent of people with chronic constipation were successfully treated with MiraLAX.
Can a child take MiraLAX?
Many pediatricians say it’s OK to give your child MiraLAX. The manufacturer’s site advises that it’s “for adults and children 17 years of age and older” and says to consult a doctor for children 16 and younger.
According to the site, the recommended daily dosage — if you’re 17 years or older — is 17 grams of MiraLAX powder dissolved in 4 to 8 ounces of a cold or warm beverage (like water, juice, or milk). The bottle comes with a convenient measuring cap. It also states that MiraLAX should not be used for longer than 7 days.
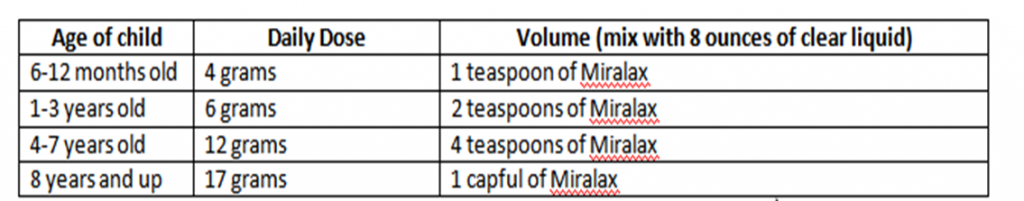
Individual clinic and physician dosage recommendations for children vary quite a bit. The dosages you may find online can seem confusing, as they’re sometimes higher than what the manufacturer recommends for adults! It’s crucial that you consult your child’s physician, who knows your child’s medical needs best.
The North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) support the use of MiraLAX in kids. And because it’s well-tolerated, easy to use, and doesn’t taste bad, it’s often one of the first treatments recommended for kids age 16 and younger. No products are FDA-approved for use in kids with constipation. Some common dosage regimen is provided below:
How safe is MiraLAX for children?
Following outcries from concerned parents that MiraLAX might be triggering psychiatric symptoms in some children, the Children’s Hospital of Philadelphia (CHOP) is currently investigating whether PEG 3350, MiraLAX’s active ingredient, causes psychiatric symptoms in children and the world is eagerly awaiting the results of this study, which was commissioned by the U.S. Food and Drug Administration.
When announcing the CHOP study back in 2015, the FDA revealed that routine testing of MiraLAX in 2008 showed trace amounts of ethylene glycol and diethylene glycol, chemicals found in antifreeze, in eight batches of MiraLAX. The FDA said these impurities resulted from the manufacturing process, and the amounts were so low that they complied with recognized safety standards. Furthermore, these chemicals were not found when batches from five PEG 3350 manufacturers were tested in 2013.
However, at this point, the scientific evidence connecting MiraLAX with psychiatric symptoms remains underwhelming. More than 100 studies have found PEG 3350 to be safe for use in children, and zero published studies have linked MiraLAX to severe or harmful side effects, psychiatric or otherwise. The most common side effects, detailed in this 2016 review article, are flatulence, abdominal pain, nausea, diarrhea, and headache.
What are the alternatives to MiraLAX for kids?
Following the MiraLAX controversy, many parents are looking for alternatives to MiraLAX for kids for their children with constipation.
The foods your child is eating can also cause constipation. A few examples of foods that might be making things worse include chips, fast food, and processed foods.
If you are one of such parents, encouraging your child to make simple dietary changes — such as eating more fiber-rich fruits and vegetables and drinking more water — can go a long way toward alleviating constipation.
According to Johns Hopkins Hospital, the following fiber-rich foods can be helpful in relieving your child’s constipation:
| Foods | Moderate fiber | High fiber |
| Bread | Whole-wheat bread, granola bread, wheat bran muffins, whole-grain waffles, popcorn | |
| Cereal | Bran cereals, shredded wheat, oatmeal, granola, oat bran | 100% bran cereal |
| Vegetables | Beets, broccoli, Brussels sprouts, cabbage, carrots, corn, green beans, green peas, acorn and butternut squash, spinach, potato with skin, avocado | |
| Fruits | Apples with peel, dates, papayas, mangoes, nectarines, oranges, pears, kiwis, strawberries, applesauce, raspberries, blackberries, raisins | Cooked prunes, dried figs |
| Meat substitutes | Peanut butter, nuts | Baked beans, black-eyed peas, garbanzo beans, lima beans, pinto beans, kidney beans, chili with beans, trail mix |
Other diet changes that may help include:
- Having your child drink more fluids, especially water
- Limiting fast foods and junk foods that are often high in fats. Offer more well-balanced meals and snacks instead.
- Limiting drinks with caffeine, such as soda and tea
- Limiting whole milk as directed by your child’s healthcare provider
It’s also a good idea to have your child eat meals on a regular schedule. Eating a meal will often cause a bowel movement within 30 to 60 minutes. Serve breakfast early. This will give your child time to have a bowel movement at home before rushing off to school.
Get more exercise
Having your child get more exercise can also help with constipation. Exercise helps with digestion. It helps the normal movements the intestines make to push food forward as it is digested. People who don’t move around much are often constipated. Have your child go outside and play rather than watch TV or do other indoor activities.
Good bowel habits
Try to get your child into a regular toilet habit. Have your child sit on the toilet at least twice a day for at least 10 minutes. Try to do this just after a meal. Be sure to make this a pleasant time. Don’t get mad at your child for not having a bowel movement. Use a reward system to make it fun. Give stickers or other small treats. Or make posters that show your child’s progress.
In some cases, these changes may not help. Or your child’s healthcare provider may detect another problem. If so, the provider may recommend using laxatives, stool softeners, or an enema. These products should only be used if recommended by your child’s provider. Do not use them without talking with your child’s provider first.