Updated WHO Technical Specifications For Blood Glucose Meter
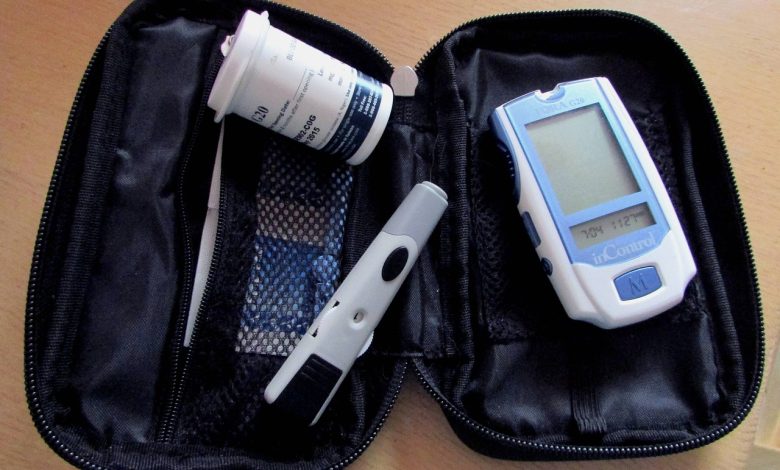
The World Health Organization (WHO) provides technical specifications and guidelines for blood glucose meters to ensure their accuracy and reliability for monitoring and managing diabetes.
These specifications are intended to help manufacturers produce high-quality devices and to guide healthcare professionals and patients in selecting appropriate blood glucose meters. Here are some of the key technical specifications recommended by WHO:
1. Accuracy: Blood glucose meters should meet specific accuracy standards, such as ISO 15197:2013, which requires that 95% of blood glucose results fall within ±15 mg/dL (0.83 mmol/L) of the reference method for glucose concentrations less than 100 mg/dL (5.56 mmol/L) and within ±15% for concentrations greater than or equal to 100 mg/dL.
2. Precision: The precision of a blood glucose meter is a measure of how consistently it provides the same result when tested with the same blood sample. WHO recommends that precision should be within certain limits specified in the relevant standards.
3. Hematocrit Range: Blood glucose meters should provide accurate results across a range of hematocrit levels, typically from 20% to 60%. Hematocrit is the measure of the volume of red blood cells in the blood.
4. Sample Size: The minimum required blood sample size for testing should be specified, and the meter should be designed to minimize the pain and discomfort associated with obtaining a blood sample.
5. Test Strip Performance: The quality and performance of the test strips used with the meter are crucial. WHO recommends that test strips meet specific criteria for accuracy, precision, and stability.
6. Operating Temperature and Humidity: Blood glucose meters should work within specified temperature and humidity ranges to ensure accurate results and device reliability.
7. Battery Life: The device should have a reasonable battery life to ensure uninterrupted use. Battery replacement should be straightforward.
8. Data Management: The meter may have data storage and retrieval capabilities, and it should be easy for users to access and review their blood glucose data.
9. Display and User Interface: The display should be clear, easy to read, and user-friendly. Instructions for use and error messages should be easily understandable.
10. Connectivity: Some modern blood glucose meters have Bluetooth or USB connectivity to transfer data to smartphones or computers. These features can be beneficial for tracking and managing diabetes.
11. Quality Control: The meter should include features for quality control, such as the ability to run control solution tests to ensure the device is functioning correctly.
12. Calibration: Some meters may require manual or automatic calibration, and the process should be straightforward for users.
It’s important for healthcare professionals and individuals with diabetes to carefully consider these technical specifications when choosing a blood glucose meter, as the accuracy and reliability of the device are critical for proper diabetes management. Additionally, these specifications can help manufacturers produce meters that meet international standards and guidelines.
| Blood Glucose Meter, handheld | ||
| i | Version No. | Two (2) |
| ii | Date of initial version | 30-Jan-20 |
| iii | Date of last modification | EMDN code/name |
| iv | Date of publication | |
| v | Prepared by | World Health Organization |
| NAME, CATEGORY, AND CODING | ||
| 1 | WHO Category / MTE Code | |
| 2 | Generic name | Glucose meter, handheld |
| 3 | Glucose, hypoglycemia, hyperglycemia, SMBG, self-monitoring, blood glucose, POCT, self-testing, diabetes | W0201060102 / Blood Glucose Meters |
| 4 | Specific type or variation (optional) | Enzymatic with variable detection methods |
| 5 | Alternative name/s (optional) | Glucometer; POCT glucose; Blood Glucose Self-Monitoring (SMBG); Haemoglucotest (HGT); Blood Glucose Meters, portable; Blood Glucose Monitors, portable; blood sugar meters, hypoglycemia detection units, portable glucose analyzers. |
| 6 | Keywords (optional) | To support diagnosis and/or monitoring of type 1 and type 2 diabetes mellitus, and/or to support diagnosis and screening for diabetes, and/or to support diagnosis of hypoglycaemia and screening for intermediate hyperglycaemia, and/or to support diagnosis of impaired fasting glucose/impaired glucose tolerance, and/or to support screening for type 2 diabetes mellitus and impaired fasting glucose/impaired glucose tolerance. |
| INTENDED USE | ||
| 7 | Detection target | Blood glucose. |
| 8 | Test purpose | Specific disorder/condition |
| 9 | Adult and pediatric | Diabetes mellitus; hypoglycemia; hyperglycemia |
| 10 | Test format | Enzymatic method, commonly either glucose oxidase or glucose dehydrogenase, with photometric, amperometric, elecrochemical or biosensor detection (the method offered/available shall be specified) |
| 11 | Specimen type | At least capillary whole blood and, preferably also venous whole blood. |
| 12 | Testing population | Equipment designed for single-patient self-testing and/or multiple-patient POC testing. Hand held portable devices, ideally training would be provided, however shall be usable without specialist training. Must be stated in instructions for use. |
| 13 | Intended user | All levels of healthcare settings (health centres, district hospitals, specialized hospitals, community based settings, health posts, home-care, etc.). |
| 14 | Level of the healthcare system | Measurement range not less than from 30 to 400 mg/dL (1.7 to 22.2 mmol/L), preferably from 20 up to 500 mg/dL (1.1 to 27,8 mmol/L). Accuracy must meet ISO-15197 standard, in particular: a) 95% of blood glucose results must be within 15% for values equal to or greater than 100 mg/dL, and 15 mg/dL for values below 100 mg/dL. b) 99% of results fall within zones A or B of the Consensus Error Grid (Parkes error grid) |
| PERFORMANCE CHARACTERISTICS | ||
| 15 | Accuracy | Analytical performance shall remain stable throughout the measurement of the analyte. The assay shall be linear across the entire measuring interval available. External Quality Assurance (EQA) at regular intervals is advisable for devices in healthcare settings (EQA watery controls only should not be preferred). |
| 16 | All levels of healthcare settings (health centers, district hospitals, specialized hospitals, community based settings, health posts, home-care, etc.). | Analytical specificity (interferences): The method shall be evaluated and demonstrate no interference from ascorbate, lipid, protein, high/low hematocrit. At least the interference list of ISO 15197:2013 shall be evaluated and demonstrated. |
| 17 | Analytical specificity | Test failure, low battery, test strip problems (i.e. expired or inserted wrong side) and malfunction messages should also be available. Error messages linked to the specific linearity range (when available). |
| 18 | Limit of detection | Not higher than 30 mg/dL or 1.7 mmol/L (better not higher than 20 mg/dL or 1.1 mmol/L) |
| 19 | Invalid/error/unreturnable rate | Typically <15% (better 10%) from the target value or less than 0.83 mmol/L for lower glucose ranges, hematocrit levels will significantly affect result (bias) (see “Test Limitations” requirement for details). |
| 20 | The trueness of measurement: bias | Repeatability (within-run variability) CV <5.0 %. Must be stated in instructions for use. |
| 21 | Trueness of measurement: bias | Precision (repeatability/reproducibility) |
| TECHNICAL AND OPERATIONAL CHARACTERISTICS | ||
| 22 | Principle of the assay | Enzymatic method (or equivalent; equivalence shall be demonstrated and clearly reported based on the standards required), commonly either glucose oxidase or glucose dehydrogenase, with photometric, amperometric, elecrochemical or biosensor detection |
| 23 | Specimen(s) stability | Specimen shall be stable for at least 15 minutes (better for at least 30 minutes) from the time to the exposure to enviroment conditions. In case of venous blood samples are used, any specific need of anti-coagulants use should be clearly stated and detailed by the manufacturer. |
| 24 | Specimen(s) volume | Sample volume at least < 15µl (in case of self-monitoring/single patient device: better if < 10µl). Manufacturer instructions for tubes and/or specific analytical instruments shall state additional requirements, if any. |
| 25 | Type of result | In the case of POCT/multiple patients devices for professionals: results are available in less than 1 minute (preferably less than 30 seconds). In case of self-monitoring/single patient device: results available in less than 30 seconds (preferable less than 10 seconds). |
| 26 | Time to result | In the case of POCT/multiple patients device for professionals: not less than 50 specimens per 8-hour working day, depending on healthcare setting. |
| 27 | End-point stability | Not applicable |
| 28 | Ease of use for POC tests only: number of steps that require precision | Single-step, easy-to-use devices. |
| 29 | Specimen throughput per operator, per hour, per 8-hour working day or per batched run | External Quality Assessment (EQA) material shall be run by a National EQA provider (when available). |
| 30 | Range of Measurement and Related Accuracy | Haematocrit acceptable range at least from 30% to 55% (better from 15% up to 65%). Device able to compensate for high haematocrit value. The manufacturer shall state ALL additional limitations, if any (i.e. blood concentrations of ascorbic acid or galactose, etc.) |
| 31 | Internal quality control | Quality control features available. Quality Control material shall be run as defined by the manufacturer. In case of self-monitoring/single patient device: automatic meter calibration check (no “coding”). In case of POCT/multiple patients device for professionals: preferably automatic meter calibration check (no “coding”) and/or calibration checks frequency and related activities clearly stated by the manufacturer (at least twice per year would be recommended). |
| 32 | Test Limitations | Compatibility with external quality control material |
| 33 | Transport stability of kit/reagents (temperature and humidity) | At least in the range from 5°C to 35°C (better if up to at least 40°C), protected against high humidity and direct sunlight. Manufacturers instructions shall state specific detail and limitations, if any. |
| 34 | Storage stability of kit/reagents (temperature and humidity) | At least in the range from 5°C to 35°C (better if up to at least 40°C), protected against high humidity and direct sunlight. Manufacturers instructions shall state specific detail and limitations, if any. |
| 35 | On-board stability of kit/reagents (temperature and humidity) | Not applicable |
| 36 | In-use stability of kit/reagents (temperature and humidity) | Not applicable |
| 37 | Transport stability for specimen collection media (temperature and humidity) | Not applicable |
| 38 | Storage stability for specimen collection media (temperature and humidity) | Not applicable |
| 39 | In-use stability for specimen collection media (temperature and humidity) | Not applicable |
| 40 | Transport stability for controls/calibrators (temperature and humidity) | Temperature and humidity stability ranges shall be clearly stated by the manufacturer, when necessary |
| 41 | Storage stability for controls/calibrators (temperature and humidity) | Temperature and humidity stability ranges shall be clearly stated by the manufacturer, when necessary |
| 42 | On-board stability for controls/calibrators (temperature and humidity) | Not applicable |
| 43 | In-use stability for controls/calibrators (temperature and humidity) | Based on manufacturer instructions |
| 44 | Shelf-life of kit/reagents upon manufacture (months) | All strips should have at least 12 12-month expiry dates (better 18 months) from the date of production (better: from the date of supply). Shelf life for reagents shall be anyway clearly stated by the manufacturer. |
| 45 | Remaining shelf-life of kit/reagents upon delivery (months) | Strips should have a minimum of 2 (better 3 or more) months shelf life after opening the strip vial. |
| INSTRUMENT PHYSICAL AND TECHNICAL CHARACTERISTICS | ||
| 46 | Size of device (Height x Width x Depth) | Handheld device (approx. average size as reference: 6 cm x 12 cm x 3 cm) |
| 47 | Weight of the device (kg) | Not greater than 0.5 kg (preferably including batteries) |
| 48 | Power requirements and characteristics | In the case of POCT/multiple patients device for professionals: not less than up to 30 days at an average of 20 tests per day and/or 600 tests in total (better: avereage of 30 tests per day and/or 900 tests in total). In case of self-monitoring/single patient device: not less than 100 tests. |
| 49 | Time to battery charge | Not higher than 3 hours |
| 50 | Battery duration | Capabilities for data transmission and storage e.g. via USB, Bluetooth, cable. In case of self-monitoring/single patient device: electronic linkage to manufacturer for data updates and/or instrument monitoring should be also preferably available. In case of POCT/multiple patients device for professionals: Availability of a digital connection to the Electronic Patient Record AND/OR data connectivity compatible with laboratory information system (LIS) and programme database to enable information sharing. Electronic linkage to manufacturer for data updates and instrument monitoring should be also preferably available. |
| 51 | Alternative charging options | Solar |
| 52 | Operating and storage conditions (temperature and humidity) | Glucometer: operating temperature range at least: 10°C – 40°C (better at least: 5°C – 45°C), humidity: 10-80% (better up to 90%) without condensation, and atmospheric pressure operating range: at least from 800 up to 1060hPa; additional altitude related operating limitations, up to at least 3000m, should be clearly stated by the manufacturer. Storage conditions range: at least -5°C – 50°C (better at least: -10°C – 55°C) and humidity 20 % – 80% RH. Test Strips packages: for high humidity (> 80%) or temperature conditions (> 40°C), the manufacturer shall guarantee that the container for strips, when provided, can be opened frequently without influencing the performance of strips/glucosemeter. |
| 53 | User interface | LCD display, simple test menu. In the case of POCT/multiple patient devices for professionals: option for connection to laboratory computer and middleware should be also available. In case of self-monitoring/single patient device: technical solutions to improve the readability for private users with limited visual capacity should be preferably available. |
| 54 | Displayed parameters | Digital display of test results. Electronic output. Audible and/or visual alert/alarm indicator at least for “low battery”, “malfunction” and/or “strips error”. Preferable: “expired strip” messages/alarm when a strip used is expired and additional alarm/warning for elevated or too low glucose levels |
| 55 | Display languages | At least 20 languages should be available including Arabic, Chinese, English, French, Russian and Spanish. Local language of the Country of sales would be also preferred, when available. |
| 56 | Built-in memory storage capacity | In case of POCT/multiple patients device for professionals: storage for at least 300 test results and 10 control results (better up to at least 500 test results and 20 control results). In case of self-monitoring/single patient device: storage for at least 100 test results (better up to at least 250 test-results for long term-storage) and, when available, 5 control results. |
| 57 | Diagnostic connectivity | Dedicated blood glucose analyzer |
| 58 | Open or Closed system | Preferably open (able to use compatible consumables of different brands) |
| 59 | Multidisease testing capabilities / Test menu availability | Dedicated blood glucose analyser |
| INFRASTRUCTURE REQUIREMENTS | ||
| 60 | Water requirements | Not applicable |
| 61 | Refrigeration or cold chain for storage of kit/reagents | Consumables shall be stored according to the Manufacturer’s instructions. |
| 62 | Refrigeration or cold chain for reconstituted reagents and controls | Consumables shall be stored according to the Manufacturer’s instructions. |
| ACCESSORIES, CONSUMABLES, SPARE PARTS AND OTHER COMPONENTS | ||
| 63 | Kit component – test/reagents/consumables (if relevant) | In addition, in the case of POCT/multiple patient devices for professionals: • Set of control solutions (at least: low, medium, and high), when necessary for calibration. In alternative and based on manufacturer instructions, the calibration materials shall be provided. • Data download cable (when necessary) In addition, in case of self-monitoring/single patient device: • When necessary: data download cable • When necessary and stated by manufacturer: quality control materials |
| Material Safety Data Sheet (MSDS) | ||
| 64 | Reagent Kit size (number of tests) | Specimen collection receptacles. A list of reagents, consumables, and accessories, including available compatible commercial products if any, shall be provided by the bidder, the distributor and/or the manufacturer. |
| 65 | Consumables required but not provided in the test kit | In case of POCT/multiple patients devices for professionals: when needed, pipettes (autopipettes and glass), gloves, eye defenders, laboratory coat and other materials could be requested. In case of self-monitoring/single patient device: not applicable |
| 66 | Other auxiliary laboratory equipment required but not provided | The kit/package size of test strips (or other compatible consumables such as cuvettes, etc.) will depend on the health-care setting and the workload. In case of self-monitoring/single patient device: single packages containing the complete material for not more than 50 tests each should be available (better if not more than 25 per each package). |
| 67 | Spare parts (if relevant) | Set of spare compatible batteries (preferably available in the local market). List of spare parts, including available compatible commercial products if any, shall be provided by the bidder, the distributor and/or the manufacturer. |
| DOCUMENTATION | ||
| 68 | Instrument operator manual | Operating and service manuals available in at least 20 languages (including Arabic, Chinese, English, French, Russian and Spanish) must include lists of important spares and accessories – with their part numbers and list of equipment and procedures required for calibration and routine maintenance. Documentation must also show recommended procedures for disposal and any probable hazards to the environment and/or community. Preferably: manufacturers should provide the translation of the operating manual in the local lanugage of the country of sales |
| 69 | Instructions for use | Required: IFU (Instructions For Use) submitted must relate to the regulatory version registered for sale and use in the country of supply. Manufacturers should provide the translation of the IFU in the local lanugage of the country of sales. In case of self-monitoring/single patient device: solutions to improve the readability for private users with limited visual capacity should be preferably available. |
| 70 | Certificate of analysis | Required. Apart from the specific product test pass report, a Certificate of Analysis must be provided by the manufacturer and must comply with the local and country requirements for the product. They shall contain the actual results obtained from final quality control for lot release performed for each lot of a product. |
| 71 | Operated by an internal battery with not any memory loss if batteries are removed. Batteries may be single use, or rechargeable (preferred) with external AC battery charger, or by USB connection. Power requirement 100-240 V (± 10%)/ 50-60Hz (country dependant) for rechargeable devices. Charger, if used, must have protection against over-voltage and over-current line conditions. If rechargeable device is provided, the operation should be preferably possible while charging. Battery should be replaceable without using any tools. Preferable: due to trasnport restrictions, the compatible batteries provided should be without Lithium | Required. The manufacturer shall supply detailed documentation for each method providing detailed and comprehensive information of a product related to (i) the health effects of exposure to the product; (ii) hazard evaluation related to the handling of the product, storage, use and disposal; (iii) measures to protect workers at risk of exposure; and (iv) emergency procedures. |
| ENVIRONMENTAL AND SAFETY REQUIREMENTS | ||
| 72 | Hazardous classification | Power requirement 100-240 V (± 10%)/ 50-60Hz (country dependent) for rechargeable devices. Some devices will use single use batteries. The device is fully automatic and it shall be provided already calibrated. Specific calibration recurrent activities (if necessary) shall be clearly stated by the manufacturer in the documentation provided. Automatic shutoff function. |
| 73 | Disposal requirements | Description of disposal techniques available and/or required, according to the hazard classification e.g. simple waste disposal, hazardous waste, incineration etc. |
| TRAINING, INSTALLATION AND UTILISATION | ||
| 74 | Pre-installation requirements (if relevant) | Not applicable |
| 75 | Requirements for installation and calibration | Manufacturers shall provide training details related to the use and maintenance of the device. Preferable: video tutorial for equipment use and maintenance shall be available on line and recorded in a CD, DVD or USB delivered with the equipment (in case of self-monitoring/single patient device: special focus should be preferably put on illiterate private users and on users with limited visual capacity). In addition, in case of POCT/multiple patients device for professionals: User training topics shall include at least: 1) Normal operating mode, 2) Daily and periodic cleaning and maintenance, 3) Basic troubleshooting, 4) Risks in the use of the equipment. Maintenance training topics shall include at least: 1) Normal operating mode, 2) Daily and periodic cleaning and maintenance, 3) Advanced troubleshooting, 4) Accessories and spare parts description and codes, 5) Warranty conditions, 6) Supplier and Manufacturer contact references. |
| 76 | Training of user/s (if relevant) | The manufacturer shall clearly document the potential source of harm in kit inserts. Classification shall be according to the Global Harmonized System (GHS) e.g. health hazard, physical hazard, or environmental hazard. More specific details will usually be contained in the Material Safety Data Sheet (MSDS) |
| WARRANTY AND MAINTENANCE | ||
| 77 | Warranty | DECOMMISSIONING |
| 78 | Preventive maintenance | The device has the capability to be cleaned and disinfected easily and to hold up under frequent disinfection.The minimal maintenance expected to be conducted by user and supplier shall be stated. Frequency of maintenance shall be based either on a fixed time period or based on the number of tests the instrument processes; this will de dictated by workload. Generally will include external cleaning of the device and change of batteries. |
| 79 | Corrective maintenance | Not applicable |
| 80 | Type of service contract (including leasing or reagent rental) | The possibility of an instrument loan should be preferably available. In case of POCT/multiple patients device for professionals: in addition to the warranty obligations, any service contract must cover labour, repair, spare parts, shipping and logistics costs and training. |
| 81 | Spare parts availability post-warranty | Availability of spare parts (but also accessories and consumables) shall be at least 5 years |
| 82 | In-country technical support availability | In addition to the warranty obligations, the manufacturer or agreed distributor should have in-country technical support available to provide adequate maintenance/repair support, when needed. |
| DECOMMISIONING | ||
| 83 | Estimated lifespan | For the product to be considered, it shall have marketing authorization for supply within the jurisdiction of a well-established medical devices regulator and comply with the corresponding pre-market requirements as described in the WHO guidance for procurement of in vitro diagnostics and related laboratory items and equipment. (see https://apps.who.int/iris/bitstream/handle/10665/255577/9789241512558-eng.pdf?sequence=1&isAllowed=y). |
| QUALITY AND REGISTRATION | ||
| 84 | Global regulatory approvals | Regulatory approval/certification |
| 85 | Free sales certificate (FSC) or certificate for exportation of medical device provided by the authority in manufacturing country. Proof of regulatory compliance, as appropriate, for example, but not limited to the following product’s risk classifications: EUROPE/CE 2017/745 conformity or equipment with CE mark according with 93/42 EEC; USA/FDA certification (Food and Drug Administration) for internal US market; CANADA/SOR/98-282 conformity certification; AUSTRALIA/TGA Conformity certification; JAPAN/ PMDA pre-market approval; etc. | A summary of current post-market information by an applicant should be provided, when available, as an indicator of product performance. Contractual agreements shall require the supplier to have procedures and agreements to support post-market activities in accordance with their role in a post-market system. Whether the supplier is a manufacturer, an authorised representative, or a distributor, each has an important role in ensuring timely feedback of complaints, the identification of non-conformity, and subsequent recall, corrections or corrective actions. |
| 86 | Standards for the manufacturer | Certified quality management system for medical devices (e.g. ISO 13485). Application of risk management to medical devices (e.g. ISO 14971). |
| 87 | WHO prequalification status | Prequalification is recommended If the test category is within the scope of WHO PQ. Please see WHO Public Reports for In Vitro Diagnostics https://extranet.who.int/pqweb/vitro-diagnostics/prequalification-reports/whopr If the test category is not within the scope of WHO PQ then not applicable. |
| 88 | Post-market surveillance | The manufacturer must provide a minimum of two (2) years warranty from the date of puchase. The warranty shall cover all delivered equipment and accessories. The date of commencement, duration of warranty period, specific exclusions/inclusions and other conditions such as maintenance support during warranty must be specified. Distrbution and after-sale services networks to be specified by the manufacturer or by the bidder at the time of the purchase. In case that the “Supplier” propose a manufacturer warranty extension, at least the following requirements shall be included: a. The warranty covers all equipment and accessories delivered. b. Specified duration including corrective maintenance. c. It includes corrective maintenance: labour and spare parts with no call limitations with the sole exclusion of damage from proven misuse. d. Corrective maintenance during warranty can be carried out at the beneficiary premise, or by mailing the broken equipment at supplier or manufacturer premise and mailing back the repaired equipment or the subtituting one to the Beneficiary premise. In the case of transportation all the costs and risks will be covered by the Supplier. e. The time elapsed between the communication of the faulty equipment and the response of the Supplier will be, within the warranty period, no more than 2 business days. f. The corrective maintenance technical service must be performed by a company based in the country of destination. g. Supplier will notify the end user in case of any equipment Recall / Field Safety Notice / Alerts as issued by the manufacturer/NRA or any other relevant authorities and organizations. |
| 89 | Field Safety Collective Actions | It is recommended that manufacturers/providers have communication channels in place to notify any corrective actions to final users, such communication channels shall be declared in the contract. |
| 90 | Standards for product performance | Compliance to the following international standards or to regional or national equivalent, (including the technical tests for safety and performance from accredited laboratory or third party). Latest version recommended but compliance to previous standards versions could be accepted: • IEC 60601-1-2 Medical electrical equipment – Part 1-2: General requirements for basic safety and essential performance – Collateral Standard: Electromagnetic disturbances – Requirements and tests • IEC 60601-1-6 Medical electrical equipment – Part 1-6: General requirements for basic safety and essential performance – Collateral standard: Usability • ISO 15197 In vitro diagnostic test systems — Requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus • ISO 22870:2016: Point-of-care testing (POCT) — Requirements for quality and competence. |
Last Update: 5th February, 2023.
Source: WHO