Thalidomide Babies: A Tragic Legacy of Medical Disaster
The term “Thalidomide babies” refers to a generation of children who were born with severe birth defects as a result of their mothers taking the drug thalidomide during pregnancy. This pharmaceutical tragedy, which unfolded in the late 1950s and early 1960s, left an indelible mark on medical history and forever changed the way drugs are tested and approved.
Thalidomide was first synthesized in Germany in the late 1950s by the pharmaceutical company Chemie Grünenthal. It was initially intended as a potential treatment for anxiety, sleeplessness, and morning sickness during pregnancy. The drug quickly gained popularity due to its sedative properties and its reputation as a safe medication for pregnant women.
In 1957, thalidomide was released in West Germany under the trade name Contergan. It was marketed as a non-addictive alternative to other sedatives and was particularly promoted to expectant mothers experiencing morning sickness. The drug’s manufacturer heavily advertised its safety, and it soon became widely prescribed across Europe and other parts of the world.
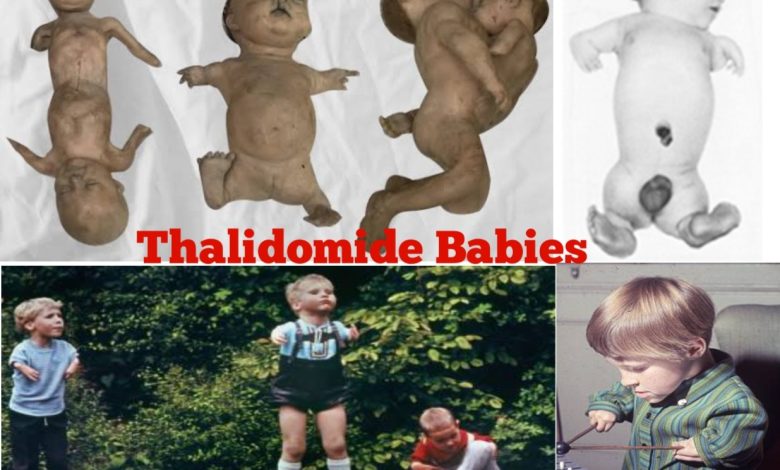
However, in the early 1960s, reports of an unusually high number of babies born with severe malformations began to emerge. These birth defects included phocomelia, a condition in which the limbs of the affected infants were severely underdeveloped or absent. Other deformities, such as internal organ abnormalities, hearing loss, and vision problems, were also observed.
Dr. Widukind Lenz, a German pediatrician, was among the first to link thalidomide to these birth defects. In 1961, he published a study detailing the association between thalidomide use during pregnancy and the occurrence of limb abnormalities in infants. This groundbreaking research served as a catalyst for further investigations into the devastating effects of the drug.
As the extent of the thalidomide disaster became apparent, countries around the world took swift action to protect their populations. By late 1961 and early 1962, thalidomide was withdrawn from the market in several countries, including Germany, the United Kingdom, Canada, and Australia. This withdrawal was accompanied by heightened public awareness and outrage, as the drug had already caused irreversible harm to thousands of children.
This article explores the tragic story of thalidomide babies, examining the causes, effects, and aftermath of this medical disaster. It also highlights the lessons learned from this event, which have greatly influenced the regulations and protocols surrounding drug testing and approval.
The Impact on Thalidomide Babies
The impact on thalidomide babies, those born with severe birth defects as a result of their mothers taking thalidomide during pregnancy, has been profound and far-reaching. These children faced numerous physical, emotional, and social challenges throughout their lives, as did their families. Let’s explore the impact on thalidomide babies in more detail:
- Physical Challenges: Thalidomide babies experienced a range of physical challenges, the most notable being limb malformations. Many infants were born with shortened or absent arms and legs, a condition known as phocomelia. These physical disabilities often required extensive medical interventions, including surgeries, prosthetics, and assistive devices, to enhance mobility and functionality. In addition to limb abnormalities, thalidomide could also cause internal organ malformations, sensory impairments (such as hearing loss or vision problems), and other physical health issues. These conditions necessitated ongoing medical care and monitoring, often requiring specialized interventions from a multidisciplinary healthcare team.
- Emotional and Psychological Impact: Thalidomide babies and their families faced significant emotional and psychological challenges. Children with visible disabilities often experienced feelings of self-consciousness, low self-esteem, and social isolation due to societal attitudes and perceptions. They may have encountered bullying or discrimination, which further exacerbated their emotional well-being. Parents of thalidomide babies also experienced a wide range of emotional responses. They grappled with guilt, grief, and feelings of responsibility for their child’s condition. Coping with the physical and emotional demands of caring for a child with significant disabilities placed additional strain on families.
- Social Challenges and Stigma: Thalidomide babies faced societal challenges and stigmatization due to their visible disabilities. In many cases, they encountered barriers to education, employment, and social participation. Limited accessibility and prejudiced attitudes often restricted their opportunities for integration and inclusion. Stigma and misconceptions surrounding thalidomide also affected the families of affected children. They often had to contend with societal judgment, ignorance, and the need to constantly advocate for their child’s rights and well-being.
- Advocacy and Support: Despite the challenges they faced, thalidomide babies and their families demonstrated remarkable resilience. They fought for recognition, compensation, and improved support systems. Advocacy groups were formed, and individuals shared their stories to raise awareness about the needs and rights of thalidomide survivors. Over time, greater awareness and understanding of disabilities have led to increased support and opportunities for thalidomide babies. Efforts have been made to improve accessibility, inclusive education, and employment opportunities for individuals with disabilities, including those affected by thalidomide.
Legal Battles and Compensation
The thalidomide tragedy led to numerous legal battles and efforts to secure compensation for the affected individuals and their families. Here is an overview of the legal aspects and compensation efforts related to thalidomide:
1. Lawsuits against Manufacturers: In the wake of the thalidomide disaster, affected families and advocacy groups initiated legal action against the drug’s manufacturer, Chemie Grünenthal, and other companies involved in its distribution. Lawsuits alleged negligence, inadequate testing, and failure to provide sufficient warnings about the potential risks associated with the drug.
These legal battles extended over several years and took place in various countries. The lawsuits aimed to hold the pharmaceutical companies accountable for the harm caused to the thalidomide babies and sought compensation for the medical expenses, ongoing care, and loss of quality of life experienced by the affected individuals.
2. Settlements and Compensation: In some cases, legal actions resulted in settlements between the plaintiffs and the pharmaceutical companies involved. These settlements were intended to provide financial support and assistance to the thalidomide survivors and their families. Compensation amounts varied depending on the jurisdiction, the severity of the disabilities, and other factors.
It is important to note that the compensation process and outcomes differed across countries. Some countries, such as Germany and the United Kingdom, established compensation funds to provide ongoing support and assistance to thalidomide survivors. In other cases, individual lawsuits resulted in monetary settlements.
3. Governmental Support and Initiatives: Many governments recognized the need to provide assistance to the thalidomide survivors. Some countries established governmental programs and initiatives to support the affected individuals and their families. These programs aimed to provide financial assistance, medical care, rehabilitation services, and other forms of support.
In addition to governmental support, advocacy groups and organizations played a crucial role in advocating for the rights and needs of thalidomide survivors. These groups worked tirelessly to raise awareness, secure compensation, and ensure that appropriate support systems were in place.
4. Lessons Learned and Regulatory Changes: The legal battles and compensation efforts related to thalidomide underscored the need for stronger regulations and more rigorous drug testing procedures. Governments and regulatory bodies implemented significant changes to drug approval processes, emphasizing the importance of thorough testing and assessment of potential risks.
Lessons Learned and Ongoing Implications
The thalidomide tragedy had profound and enduring implications that continue to shape the field of medicine and pharmaceutical regulation. The lessons learned from this devastating event have led to significant changes in drug testing, approval processes, and patient safety. Here are some of the key lessons learned and ongoing implications of the thalidomide disaster:
1. Stricter Regulations and Drug Approval Processes: The thalidomide catastrophe highlighted the need for more rigorous regulations and robust drug approval processes. Governments and regulatory agencies worldwide have implemented stricter guidelines and protocols to ensure thorough testing and evaluation of potential risks before drugs are brought to market. This includes more extensive preclinical and clinical trials, stricter monitoring, and improved reporting systems.
2. Focus on Patient Safety and Informed Consent: The thalidomide tragedy emphasized the paramount importance of patient safety and informed consent in medical research and drug development. The incident highlighted the need for clear communication of potential risks and benefits to patients, especially vulnerable populations like pregnant women. Today, informed consent is a fundamental principle in clinical trials and medical interventions, ensuring that patients are fully aware of the potential risks associated with any treatment.
3. Regulatory Agencies and Pharmacovigilance: The thalidomide disaster led to the establishment and strengthening of regulatory agencies dedicated to evaluating the safety and efficacy of pharmaceutical products. Agencies like the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and others now play a critical role in reviewing and approving drugs, monitoring their use, and implementing post-marketing surveillance to identify and address potential adverse effects.
4. Ethical Considerations in Medical Research: Thalidomide prompted a significant shift in ethical considerations in medical research. It highlighted the need to protect vulnerable populations, particularly pregnant women and their unborn children, from potential harm. Ethical guidelines and committees have been established to ensure that research involving human subjects adheres to strict ethical principles, minimizing risks and safeguarding the welfare of participants.
5. Improved Drug Labeling and Warning Systems: Thalidomide underscored the importance of clear and accurate drug labeling and warning systems. Today, pharmaceutical companies are required to provide comprehensive information on potential risks, contraindications, and side effects associated with their products. This enables healthcare professionals and patients to make informed decisions about drug use and minimize the chances of adverse effects.
6. Impact on Thalidomide Use and Repurposing: The thalidomide tragedy initially resulted in a global ban on the use of the drug. However, over time, researchers discovered potential therapeutic benefits in certain medical conditions, such as multiple myeloma and certain autoimmune disorders. Strict regulations are now in place to ensure the safe and controlled use of thalidomide in specific circumstances where the benefits outweigh the risks. The case of thalidomide has served as a cautionary example of the importance of careful evaluation and monitoring when repurposing drugs.
7. Patient Advocacy and Support: The thalidomide disaster brought the issues faced by thalidomide survivors and their families to the forefront, leading to increased advocacy and support initiatives. Today, there are numerous organizations and support networks dedicated to promoting the rights, well-being, and inclusion of individuals with disabilities. Thalidomide survivors and their advocates continue to play a crucial role in raising awareness, advocating for improved accessibility and healthcare, and pushing for fair compensation and support systems.
Conclusion
The story of thalidomide babies serves as a stark reminder of the devastating consequences that can arise from the inadequate testing and regulation of pharmaceutical drugs. The tragedy sparked significant changes in drug approval processes and prioritized patient safety. The resilience of thalidomide survivors and their advocates has led to greater awareness, support, and understanding for individuals with disabilities.
While the thalidomide disaster cannot be undone, it stands as a testament to the importance of rigorous testing, ethical considerations, and ongoing vigilance in the development and use of medical treatments. By learning from the mistakes of the past, we can strive to ensure that such tragedies are not repeated and that the welfare of patients remains paramount in the advancement of medical science and therapeutics.