Teva Secures Early Launch of Generic Vivitrol After Settling Patent Case with Alkermes
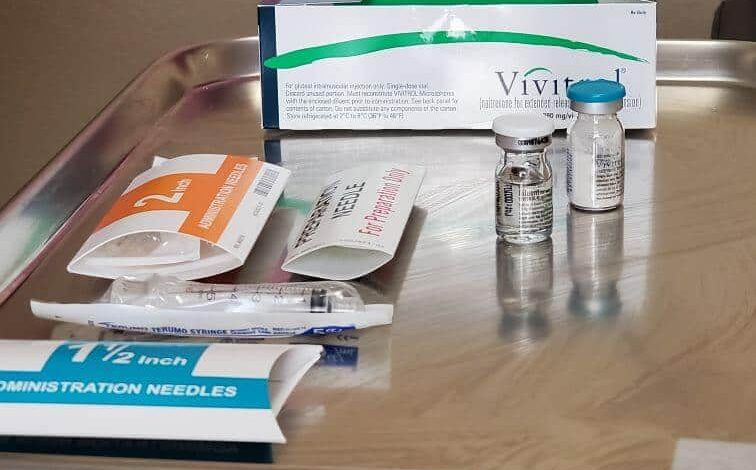
In a significant legal development, Teva Pharmaceuticals has reached a settlement with Alkermes, the manufacturer of Vivitrol, a medication used for opioid and alcohol dependence. This settlement grants Teva the license to introduce its generic version of Vivitrol several years before the drug’s patent expiration, reshaping the competitive landscape in this critical therapeutic area.
Under the terms of the agreement, Teva is granted the right to launch its generic rendition of Vivitrol on or before January 15, 2027, subject to specific customary conditions. This early market entry date, which falls two years prior to Vivitrol’s anticipated patent expiration in 2029, significantly accelerates the introduction of a more affordable alternative to this vital medication.
While specific financial details of the settlement remain undisclosed, the agreement carries profound implications for both Teva and Alkermes. The settlement effectively resolves the patent infringement lawsuit that Alkermes initiated against Teva in 2020, which subsequently progressed to a trial in March. As a result of this resolution, the two companies will jointly request the U.S. District Court for the District of New Jersey to dismiss the litigation, as outlined in Alkermes’ official statement.
Vivitrol, notable for its extended-release intramuscular injectable formulation, received its initial FDA approval in 2006 for the treatment of alcohol dependence. The medication’s approval expanded in 2010 to encompass opioid dependence as well, significantly boosting its commercial potential. However, Alkermes faced scrutiny for its marketing practices amid the backdrop of the opioid epidemic. An investigation led by then-Senator Kamala Harris in 2017 shed light on the company’s “aggressive lobbying and marketing campaign.” Subsequently, the FDA’s Office of Prescription Drug Promotion issued a stern warning letter to Alkermes for omitting critical safety risks in one of its advertisements.
Despite these controversies, Vivitrol has continued to be a substantial revenue generator for Alkermes. In the previous year, the medication generated approximately $379.5 million in sales, reflecting a notable 10% increase from 2021. Alkermes has projected sales for the full year of 2023 to range between $380 million and $410 million, further underlining the medication’s financial significance within the company’s portfolio.
Teva’s strategic maneuver to secure an early license for its generic version of Vivitrol signifies a significant shift in the pharmaceutical landscape, allowing for increased accessibility and potentially reducing the financial burden on patients seeking treatment for opioid and alcohol dependence. This settlement not only showcases Teva’s commitment to delivering cost-effective healthcare solutions but also highlights the broader complexities of pharmaceutical patent disputes and the dynamic interplay between drug manufacturers in addressing critical public health challenges.