Mosegor Vita: Ingredients, Uses, Dosage, Side Effects, Warnings
What is Mosegor Vita?
Mosegor Vita is a combination medication containing pizotifen and vitamin B-complex. Pizotifen belongs to the class of antamines and is related to cyproheptadine. It is a potent serotonin and tryptamine antagonist that has been used for migraine prevention for many years. It exhibits weak anticholinergic, antihistamine, and antikinin actions in addition to sedative and appetite-stimulating properties.
Vitamin B complex is a product that’s composed of eight B vitamins. B vitamins play a vital role in maintaining good health and well-being. As the building blocks of a healthy body, B vitamins have a direct impact on your energy levels, brain function, and cell metabolism. Vitamin B complex may help prevent infections and help support or promote: cell health.
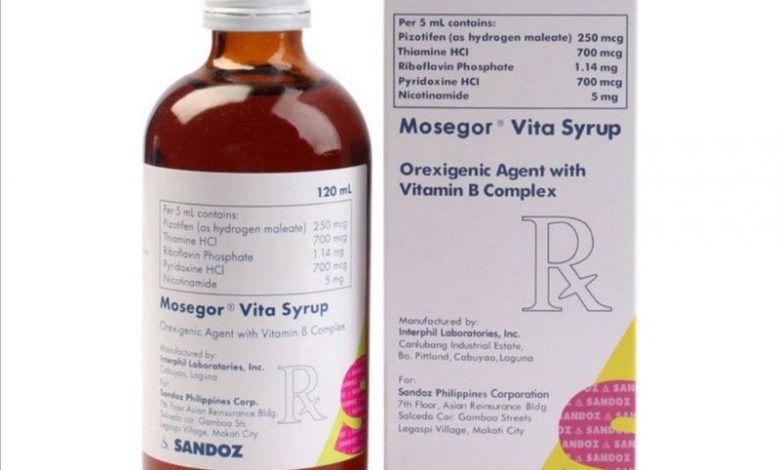
Each Capsule of Mosegor Vita contains the following active ingredients; Pizotifen 0.5 mg, thiamine (vitamin B1) 3 mg, riboflavin (vitamin B2) 3.2 mg, pyridoxine (vitamin B6) 2.4 mg, nicotinamide 19 mg. Per 5 mL syrup: Pizotifen 0.25 mg, thiamine (vitamin B1) 0.7 mg, riboflavin (vitamin B2) 1.14 mg, pyridoxine (vitamin B6) 0.7 mg, nicotinamide 5 mg.
Mosegor Vita is manufactured by Novartis Healthcare Philippines, Inc. and it is available in capsules, and syrup x 60 ml and, 120 ml.
What is Mosegor Vita used for?
Mosegor Vita has an appetite-stimulating action suitable for increasing body weight in underweight anorectic patients. The compound is well-tolerated, permitting treatment of anorexia both in children and adults.
Mosegor Vita is indicated for anorexia of somatic or psychogenic origin in underweight patients, in whom prevention of vitamin B deficiency secondary to impaired dietary intake or absorption (e.g. as a consequence of antibiotics or other drug therapy interfering with absorption or utilization of B-group vitamins).
How to take Mosegor Vita
The recommended dosage of Mosegor Vita is as follows:
For Syrup: Children: Small initial dose should be gradually increased to an average maintenance dosage of 0.025 mg. Pizotifen per kg. body weight daily e.g., 2 – 6 years: 10 – 20 mL daily in 2 or 3 divided doses or as directed by the physician.
Adults: Starting with 10 mL syrup per day, dosage should be progressively increased up to 10 mL syrup three times daily.
Duration of therapy: as directed by the physician.
For Capsules: Adults: Starting with 1 capsule per day, dosage should be progressively increased to 1 capsule thrice daily.
Duration of therapy: as directed by the physician.
Do not suddenly stop taking this medication without consulting your doctor. “Rebound headaches” may occur when the drug is suddenly stopped. Your dose should be gradually decreased over a 2-week period.
When used for an extended period, this medication may not work as well and may require different dosing. Talk with your doctor if this medication stops working well.
What happens if I overdose?
Symptoms of Mosegor Vita overdose include drowsiness, nausea, hypotension, dizziness, excitatory states (in children), and coma.
Can pregnant and breastfeeding mothers take Mosegor Vita?
No, pregnant women and breastfeeding women should avoid Mosegor Vita. This medication should not be also used by anyone hypersensitive to any of its ingredients and children less than 1 year old. Patients with narrow-angle glaucoma or urinary retention (e.g. in prostatic enlargement) should avoid this medication. This medication may slow reactions when driving or operating machinery.
What are the side effects of Mosegor Vita?
Common Side Effects
- Drowsiness
- Weight gain
- Tiredness
- Nausea
- Headache
- Dry mouth may occur
If any of these effects persist or worsen, tell your doctor or pharmacist promptly.
Remember that your doctor has prescribed this medication because he or she has judged that the benefit to you is greater than the risk of side effects. Many people using this medication do not have serious side effects.
Tell your doctor right away if any of these unlikely but serious side effects occur:
- eye pain
- dark urine
- persistent nausea and vomiting
- severe stomach and abdominal pain
- yellowing eyes and skin
- vision changes
- mental and mood changes (e.g., confusion, depression, nervousness)
- swelling of the ankles and feet
- decreased sexual ability
A very serious allergic reaction to this drug is rare. However, seek immediate medical attention if you notice any symptoms of a serious allergic reaction, including:
- rash
- itching/swelling (especially of the face, tongue, and throat)
- severe dizziness
- trouble breathing
This is not a complete list of possible side effects. If you notice other effects not listed above, contact your doctor or pharmacist.
In the US –
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch.
In Canada – Call your doctor for medical advice about side effects. You may report side effects to Health Canada at 1-866-234-2345.
What medication interacts with Mosegor Vita?
Mosegor Vita can interact with sedatives, hypnotics, antihistamines including certain common cold preparations, and alcohol.





