Suflave: Uses, Benefits, Dosage, Side Effects, How to Prepare, Interactions
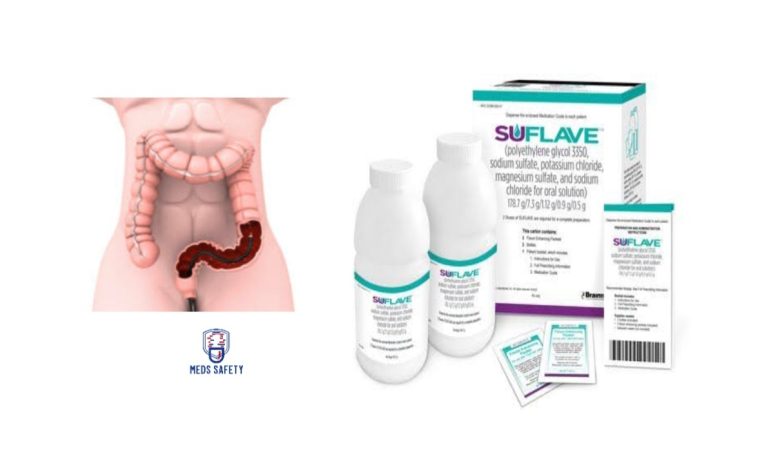
Suflave is a newly approved medication that serves as an osmotic laxative, specifically designed for the purpose of cleansing the colon prior to a colonoscopy procedure in adult patients. It contains a combination of active ingredients, including polyethylene glycol 3350, sodium sulfate, potassium chloride, magnesium sulfate, and sodium chloride, which work together to facilitate effective bowel preparation.
The primary indication for Suflave is to ensure a clear and thorough cleansing of the colon, which is essential for accurate visualization during a colonoscopy. By promoting bowel movements and increasing the volume of water in the gastrointestinal tract, Suflave aids in the elimination of fecal matter and debris, allowing for improved examination and detection of any abnormalities or lesions in the colon.
The use of Suflave is limited to adults, as it is specifically indicated for colonoscopy preparation in this patient population. It is important to note that Suflave is intended for short-term use only, and its primary function is to assist with bowel cleansing prior to the colonoscopy procedure.
Suflave is available in the form of an oral solution. Each package of Suflave consists of two bottles and two flavor-enhancing packets, designed to improve the taste of the solution.
Each bottle of Suflave oral solution contains the following active ingredients:
• Polyethylene glycol 3350: 178.7 g
• Sodium sulfate: 7.3 g
• Potassium chloride: 1.12 g
• Magnesium sulfate: 0.9 g
• Sodium chloride: 0.5 g
In addition to the active ingredients, the bottle also contains lemon-lime flavoring, which helps to enhance the taste of the solution and make it more palatable for patients.
It is important to note that the specific concentrations of the active ingredients mentioned above are based on the formulation of Suflave approved in the United States. Dosage forms and strengths of medications can vary by country or region, so it is always essential to refer to the specific product labeling or consult with a healthcare professional for accurate and up-to-date information regarding the available strengths and formulations of Suflave in your location.
How to Use Suflave
Dosage and administration instructions for Suflave are as follows:
Preparation and Administration:
• Two doses of Suflave are required for complete colonoscopy preparation.
• Each dose consists of one bottle of Suflave oral solution and one flavor-enhancing packet.
• Before ingestion, each bottle of Suflave must be reconstituted with water as per the instructions provided.
• After consuming each dose of Suflave, an additional 16 ounces (approximately 473 milliliters) of water must be consumed.
• It is important to stop consuming all fluids at least 2 hours before the scheduled colonoscopy procedure.
Recommended Dosage and Administration:
The recommended regimen for colonoscopy preparation using Suflave is the Split-Dose (two-day) regimen, which involves the following steps:
Day 1, Dose 1 (Evening before Colonoscopy):
• In the evening before the scheduled colonoscopy, take one bottle of Suflave oral solution, mixed with water as instructed, and the accompanying flavor-enhancing packet.
Day 2, Dose 2 (Morning of the Colonoscopy):
• On the day of the colonoscopy, approximately 5 to 8 hours before the procedure (but no sooner than 4 hours from starting Dose 1), take one more bottle of Suflave oral solution, mixed with water as directed, along with the flavor-enhancing packet.
For comprehensive information on the preparation process before a colonoscopy and the administration of the specific dosage regimen, it is advised to refer to the full prescribing information provided with Suflave or consult with your healthcare provider.
Who should not take Suflave?
Suflave has specific contraindications that should be taken into consideration. These contraindications are as follows:
1. Gastrointestinal obstruction or ileus: Suflave is contraindicated in individuals who have a known or suspected gastrointestinal obstruction or ileus. These conditions involve a blockage or obstruction in the gastrointestinal tract, which can interfere with the passage of the oral solution and potentially worsen the underlying condition.
2. Bowel perforation: Suflave should not be used in patients with a known or suspected bowel perforation. Bowel perforation refers to a rupture or hole in the wall of the gastrointestinal tract, and the use of Suflave in such cases may aggravate the condition or lead to complications.
3. Toxic colitis or toxic megacolon: Suflave is contraindicated in individuals with toxic colitis or toxic megacolon. These conditions involve severe inflammation of the colon, which can lead to complications such as perforation or dilatation. The use of Suflave in these cases may exacerbate the inflammation and worsen the patient’s condition.
4. Gastric retention: Suflave should not be used in patients with gastric retention, which refers to the inability of the stomach to empty its contents properly. Administration of Suflave in such cases may further delay gastric emptying and potentially cause complications.
5. Hypersensitivity: Suflave is contraindicated in individuals who have a known hypersensitivity or allergic reaction to any of the ingredients present in the medication. It is important to review the full list of ingredients and consult with a healthcare professional to identify any potential hypersensitivity before using Suflave.
It is crucial to inform your healthcare provider about any existing medical conditions or allergies you may have before starting treatment with Suflave. This will help ensure that the medication is safe and appropriate for your specific situation.
Can a breastfeeding mother take Suflave?
there is a lack of data on the presence of Suflave in human or animal milk, as well as its effects on the breastfed child and milk production, it is, therefore challenging to determine the potential risks or benefits associated with its use during lactation. Therefore, the decision to use Suflave while breastfeeding should be made after considering the developmental and health benefits of breastfeeding for the child, as well as the clinical need for Suflave in the mother.
Side Effects
The most commonly reported side effects associated with the use of Suflave include:
1. Nausea: Some patients may experience a sensation of unease or discomfort in the stomach, often accompanied by a tendency to vomit.
2. Abdominal distension: This refers to a feeling of fullness or bloating in the abdominal area, which may be caused by increased gas or fluid accumulation.
3. Vomiting: Some individuals may experience the act of forcefully expelling the contents of the stomach through the mouth.
4. Abdominal pain: Patients may experience discomfort, cramping, or pain in the abdominal region.
5. Headache: Some individuals may experience pain or discomfort in the head, often described as aching or throbbing.
It is important to note that these adverse reactions were reported in clinical studies and were observed in more than 2% of patients using Suflave. However, the actual incidence of these reactions may vary among individuals.
If you experience any of these adverse reactions or have concerns about the medication, it is recommended to contact your healthcare provider. They can provide guidance, evaluate the severity of the symptoms, and determine if any further action is necessary.
Additionally, Suflave can also cause serious side effects and has certain warnings and precautions that should be taken into consideration. These include:
1. Risk of fluid and electrolyte abnormalities: Adequate hydration is important during the use of Suflave. It is essential to assess concurrent medications and consider laboratory assessments before and after each use to monitor fluid and electrolyte levels.
2. Cardiac arrhythmias: In patients at increased risk of cardiac arrhythmias, it is advisable to consider performing pre-dose and post-colonoscopy electrocardiograms (ECGs) as part of the monitoring process.
3. Seizures: Caution should be exercised in patients with a history of seizures or those at an increased risk of seizures. This includes individuals who are taking medications that lower the seizure threshold. Close monitoring is recommended in such cases.
4. Colonic mucosal ulcerations: When interpreting colonoscopy findings, it is important to consider the potential for colonic mucosal ulcerations in patients with known or suspected inflammatory bowel disease.
5. Patients with renal impairment or taking concomitant medications affecting renal function: Extra caution should be taken in patients with renal impairment or those taking medications that may impact renal function. Adequate hydration and laboratory testing should be considered in these individuals.
6. Suspected gastrointestinal (GI) obstruction or perforation: It is important to rule out the presence of GI obstruction or perforation before administering Suflave to prevent any potential complications.
7. Patients at risk for aspiration: During the administration of Suflave, patients at risk for aspiration should be closely observed to minimize the risk of aspiration-related complications.
8. Hypersensitivity reactions, including anaphylaxis: Patients should be informed about the possibility of hypersensitivity reactions, including severe allergic reactions such as anaphylaxis. If any symptoms occur, immediate medical care should be sought.
It is crucial to discuss these warnings and precautions with your healthcare provider before using Suflave. They can provide personalized advice and guidance based on your specific medical history and individual risk factors.
Interactions
There are certain drugs that may increase the risk of fluid and electrolyte imbalance when used concomitantly with Suflave. It is important to be aware of these potential drug interactions. Some examples of medications that can increase the risk of fluid and electrolyte imbalance when used with Suflave include:
1. Diuretics: Diuretic medications, such as loop diuretics (e.g., furosemide) or thiazide diuretics (e.g., hydrochlorothiazide), increase urine production and can affect fluid and electrolyte balance. Concurrent use with Suflave may further contribute to fluid loss and electrolyte abnormalities.
2. Antihypertensive medications: Some antihypertensive medications, such as angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), may have a diuretic effect or can affect fluid and electrolyte balance. When used together with Suflave, the risk of fluid and electrolyte imbalance may be increased.
3. Nonsteroidal anti-inflammatory drugs (NSAIDs): NSAIDs, including medications like ibuprofen or naproxen, can interfere with renal function and may contribute to fluid retention or electrolyte disturbances. Concurrent use with Suflave may further increase the risk of fluid and electrolyte imbalance.
4. Certain medications affecting renal function: Medications that impact renal function, such as some antibiotics (e.g., aminoglycosides) or antiviral drugs (e.g., acyclovir), may affect fluid and electrolyte balance. When used concomitantly with Suflave, the risk of fluid and electrolyte abnormalities may be heightened.
It is important to inform your healthcare provider about all the medications you are currently taking, including prescription drugs, over-the-counter medications, and any herbal or dietary supplements. They can assess the potential drug interactions and make appropriate recommendations to manage the risk of fluid and electrolyte imbalance.
Your healthcare provider will consider the potential benefits and risks of using Suflave in conjunction with other medications and may adjust the dosages or provide additional monitoring to ensure the safe and effective use of all medications involved.