ReVive: The Second OTC Naloxone Spray Approved by FDA

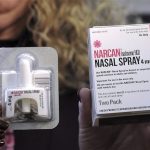
The U.S. Food and Drug Administration (FDA) has taken a significant step forward in addressing the opioid crisis by approving RiVive, an over-the-counter (OTC) naloxone hydrochloride nasal spray. Naloxone is a powerful tool that rapidly reverses the effects of opioid overdose, making it a critical asset in protecting public health. With this approval, naloxone becomes more widely accessible, empowering individuals to intervene in opioid emergencies without the need for a prescription.
FDA Commissioner Robert M. Califf, M.D., expressed the agency’s commitment to prioritizing access to naloxone products. He emphasized the importance of making naloxone available without prescription, recognizing its potential to save lives during overdose situations. This move encourages other manufacturers to explore nonprescription development programs for naloxone products, further expanding the availability of life-saving medications.
The approval of RiVive was based on comprehensive data demonstrating its efficacy and safety. Clinical studies revealed that RiVive achieves similar levels of effectiveness as approved prescription naloxone products when reaching the bloodstream. Moreover, consumers have shown the ability to use the drug safely and effectively without the direct supervision of a healthcare professional.
Bobby Mukkamala, M.D., the chair of the American Medical Association Substance Use and Pain Care Task Force, welcomed the FDA’s decision, stressing that every measure to increase the accessibility of safe and effective opioid overdose-reversal medications will undoubtedly save lives. He urged other naloxone manufacturers to swiftly submit their applications for nonprescription versions, highlighting the urgency of the opioid crisis.
Prior to the approval of RiVive, the FDA had already granted approval to the first nonprescription naloxone nasal spray product in March 2023 and the first generic nonprescription naloxone nasal spray product in July 2023. These approvals mark significant progress in making naloxone more accessible to the general public.
Harm Reduction Therapeutics, the manufacturer of RiVive, was granted approval for its contribution to tackling the opioid crisis and providing an essential tool for emergency opioid overdose treatment.
The approval of RiVive as an OTC product is a vital step in combatting the opioid epidemic, increasing access to naloxone, and empowering individuals to intervene promptly in opioid overdose emergencies, potentially saving countless lives. The FDA’s continued commitment to prioritizing access to naloxone products signals a collective effort to address this public health challenge effectively.