Tribedoce: Side Effects, Dosage, Uses, and Review

Tribedoce is a Mexican brand of Cyanocobalamin (vitamin B12), a man-made form of vitamin B12 used to prevent and treat low blood levels of this vitamin. Most people get enough vitamin B12 from their diet. Vitamin B12 is important to maintain the health of your metabolism, blood cells, and nerves.
Tribedoce injection is used to treat and prevent a lack of vitamin B12 that may be caused by any of the following: pernicious anemia (lack of a natural substance needed to absorb vitamin B12 from the intestine); certain diseases, infections, or medications that decrease the amount of vitamin B12 absorbed from food; or a vegan diet (strict vegetarian diet that does not allow any animal products, including dairy products and eggs).
Lack of vitamin B12 may cause anemia (condition in which the red blood cells do not bring enough oxygen to the organs) and permanent damage to the nerves. Tribedoce injection also may be given as a test to see how well the body can absorb vitamin B12. Tribedoce injection is in a class of medications called vitamins. Because it is injected straight into the bloodstream, it can be used to supply vitamin B12 to people who cannot absorb this vitamin through the intestine.
How should this medicine be used?
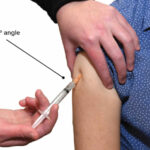
Cyanocobalamin comes as a solution (liquid) to be injected into a muscle or just under the skin. It is usually injected by a healthcare provider in an office or clinic. You will probably receive Tribedoce injection once a day for the first 6-7 days of your treatment. As your red blood cells return to normal, you will probably receive the medication every other day for 2 weeks, and then every 3-4 days for 2-3 weeks. After your anemia has been treated, you will probably receive the medication once a month to prevent your symptoms from coming back.
Tribedoce injection will supply you with enough vitamin B12 only as long as you receive injections regularly. You may receive cyanocobalamin injections every month for the rest of your life. Keep all appointments to receive cyanocobalamin injections even if you feel well. If you stop receiving cyanocobalamin injections, your anemia may return and your nerves may be damaged.
What side effects can Tribedoce injection cause?
Tribedoce injection may cause side effects. Tell your doctor if either of these symptoms is severe or does not go away:
• diarrhea
• feeling as if your entire body as swollen
Some side effects can be serious. The following symptoms are uncommon, but if you experience any of them, call your doctor immediately:
• muscle weakness, cramps, or pain
• leg pain
• extreme thirst
• frequent urination
• confusion
• shortness of breath, especially when you exercise or lie down
• coughing or wheezing
• fast heartbeat
• extreme tiredness
• swelling of the arms, hands, feet, ankles or lower legs
• pain, warmth, redness, swelling or tenderness in one leg
• headache
• dizziness
• red skin color, especially on the face
• hives
• rash
• itching
• difficulty breathing or swallowing
Tribedoce injection may cause other side effects. Call your doctor if you have any unusual problems while taking this medication.
Tribedoce Injections Safety Information
Tribedoce injections are generally considered to be very safe. However, in very rare cases, some people can experience side effects caused by allergic reactions or sensitivity.
Keep all appointments with your doctor and the laboratory. Your doctor will order certain lab tests to check your body’s response to cyanocobalamin injection.
It is important for you to keep a written list of all of the prescription and nonprescription (over-the-counter) medicines you are taking, as well as any products such as vitamins, minerals, or other dietary supplements. You should bring this list with you each time you visit a doctor or if you are admitted to a hospital. It is also important information to carry with you in case of emergencies.