Ngenla: Uses, Benefits, Dosage, Side Effects, Warnings
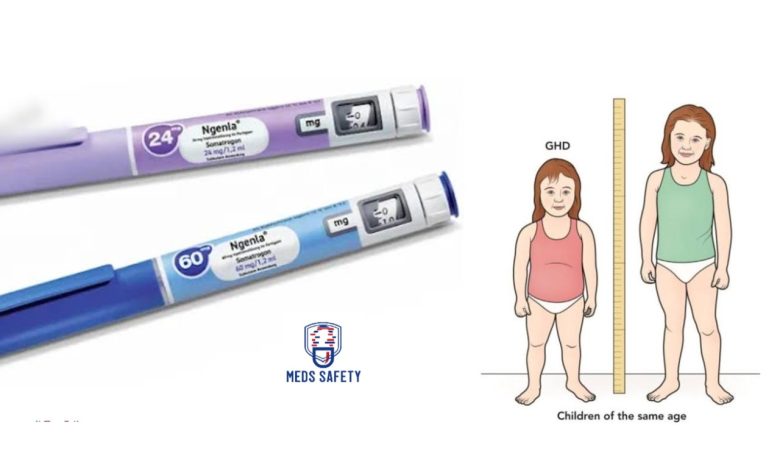
Ngenla (somatrogon-ghla) is a highly advanced and specialized human growth hormone analog that has been specifically developed for the treatment of pediatric patients aged 3 years and older. It is primarily indicated for individuals who are experiencing growth failure as a result of inadequate secretion of their own natural growth hormone.
Growth failure can occur due to various factors, and one of the primary causes is the insufficient production or secretion of endogenous growth hormone, which is responsible for normal growth and development in children. Ngenla aims to address this deficiency by providing a synthetic form of growth hormone that mimics the actions of the natural hormone.
By administering Ngenla, healthcare professionals aim to supplement the deficient growth hormone levels in pediatric patients, thereby stimulating growth and improving overall development. The use of Ngenla is intended to help these children reach a more appropriate height and catch up to their peers in terms of physical growth.
It’s important to note that Ngenla is specifically designed and approved for use in pediatric patients aged 3 years and older. This age range ensures that the children have undergone appropriate diagnostic evaluations and have been identified as having growth failure due to insufficient endogenous growth hormone secretion.
As with any medication, the use of Ngenla should be closely monitored by healthcare professionals experienced in managing pediatric growth disorders. Regular assessments of growth parameters, such as height and weight, as well as other relevant clinical markers, are necessary to determine the appropriate dosage and monitor the response to treatment.
Benefits
Ngenla offers potential benefits that can help minimize the disease management burden associated with growth hormone deficiency and improve the quality of life for affected children and their families. Here are some ways in which Ngenla can contribute to these improvements:
1. Simplified Treatment Schedule: Ngenla is administered once weekly, compared to daily injections required with some other growth hormone therapies. This less frequent dosing schedule can reduce the treatment burden for patients and their families, as well as improve convenience and adherence to the treatment regimen.
2. Improved Treatment Adherence: The simplified dosing schedule of Ngenla can potentially enhance treatment adherence. By requiring fewer injections, Ngenla may help reduce treatment fatigue and make it easier for patients and caregivers to follow the prescribed treatment plan consistently. Improved adherence can lead to better treatment outcomes and optimized growth in children with growth hormone deficiency.
3. Enhanced Quality of Life: By effectively addressing growth hormone deficiency, Ngenla can contribute to improved physical development and growth in affected children. Achieving optimal growth can positively impact a child’s overall quality of life, including their self-esteem, social interactions, and participation in daily activities. It can also reduce the emotional burden associated with delayed growth and potential stigmatization.
4. Family Support and Well-being: The simplified treatment regimen with Ngenla can relieve some of the stress and burden on families caring for children with growth hormone deficiency. It can reduce the time and effort required for administration and provide families with more flexibility and freedom in managing their daily routines. This, in turn, can improve the overall well-being and satisfaction of both the child and their caregivers.
5. Positive Treatment Outcomes: With improved treatment adherence and consistent therapy, Ngenla has the potential to yield positive treatment outcomes in terms of growth and development. By effectively addressing growth hormone deficiency, Ngenla can support the attainment of appropriate height and body composition, contributing to improved health and well-being in affected children.
It is important to note that the actual impact on quality of life and treatment adherence may vary among individuals. Consulting with healthcare professionals and following their guidance and monitoring are crucial to achieving the desired benefits of Ngenla in managing growth hormone deficiency.
Dosage forms
Ngenla is available in the form of a prefilled pen for injection. There are two different strengths of Ngenla prefilled pens:
1. 24 mg/1.2 mL (20 mg/mL) prefilled pen: This strength of Ngenla delivers a dose in 0.2 mg increments. The prefilled pen contains a total of 24 mg of Ngenla in 1.2 mL of solution, with a concentration of 20 mg/mL. The pen is designed for single-patient use, meaning it is intended for individual patients and should not be shared.
2. 60 mg/1.2 mL (50 mg/mL) prefilled pen: This strength of Ngenla delivers a dose in 0.5 mg increments. The prefilled pen contains a total of 60 mg of Ngenla in 1.2 mL of solution, with a concentration of 50 mg/mL. Similar to the 24 mg strength, this pen is also intended for single-patient use.
The prefilled pen design allows for easy and convenient administration of Ngenla. It eliminates the need for vials, syringes, and reconstitution, making it more user-friendly for healthcare professionals and patients alike.
It’s worth noting that the choice of strength and dosing regimen for Ngenla will be determined by the prescribing healthcare professional based on the individual needs and specific growth hormone requirements of the pediatric patient. The dosage may vary depending on factors such as the patient’s age, weight, and response to treatment. Close monitoring and regular follow-up with the healthcare provider are crucial to ensure optimal dosing and therapeutic outcomes.
How to Administer Ngenla
The dosage and administration guidelines for Ngenla are as follows:
1. Supervision by a Healthcare Provider: Ngenla treatment should be overseen by a healthcare provider who has expertise in diagnosing and managing pediatric patients with growth hormone deficiency.
2. Administration Method: Ngenla is administered via subcutaneous injection once weekly. The injections should be given on the same day each week, and the injection sites should be rotated weekly. The abdomen, thighs, buttocks, or upper arms can be used as injection sites.
3. Recommended Dosage: The recommended dosage of Ngenla is 0.66 mg/kg based on the patient’s actual body weight. This dosage is administered once weekly.
4. Individualized Dosage: The dosage of Ngenla should be individualized for each patient based on their growth response. The healthcare provider will assess the patient’s progress and adjust the dosage as needed to achieve the desired growth outcomes.
5. Switching from Daily Growth Hormone: Patients who are switching from daily growth hormone therapy can initiate treatment with Ngenla on the day following their last daily injection. The healthcare provider will guide the transition process.
6. Multiple Injections for Complete Dose: If the prescribed dose of Ngenla requires more than one injection, each injection should be administered at a different injection site. This helps in spreading out the injections and minimizing discomfort at any single site.
It’s essential to adhere to the prescribed dosage and administration instructions provided by the healthcare provider. Regular follow-up appointments will be necessary to monitor the patient’s growth progress and make any necessary adjustments to the treatment regimen.
Side effects
When using Ngenla, some patients may experience adverse reactions. The following adverse reactions have been reported in at least 5% of patients treated with Ngenla:
1. Injection site reactions: These include redness, swelling, pain, or itching at the site of injection. It is common to experience some mild local reactions at the injection site.
2. Nasopharyngitis: This refers to inflammation of the nasal passages and throat, commonly known as the common cold. Symptoms may include a runny or stuffy nose, sneezing, sore throat, and cough.
3. Headache: Patients may experience mild to moderate headaches as a potential side effect of Ngenla treatment. Monitoring and managing headaches can help alleviate discomfort.
4. Pyrexia: Pyrexia refers to an elevated body temperature or fever. Fever can be a common side effect associated with Ngenla treatment.
5. Anemia: Anemia is a condition characterized by a decreased number of red blood cells or hemoglobin, leading to reduced oxygen-carrying capacity in the blood. Anemia may occur as an adverse reaction to Ngenla treatment.
6. Cough: Some patients may experience coughing as a side effect. Monitoring the severity and duration of the cough is important to ensure it does not become persistent or bothersome.
7. Vomiting: Nausea and vomiting may occur in some patients as a result of Ngenla treatment. Monitoring the frequency and severity of vomiting is important.
8. Hypothyroidism: Hypothyroidism is a condition where the thyroid gland does not produce enough thyroid hormone, leading to various symptoms related to a slow metabolism.
9. Abdominal pain: Abdominal pain refers to discomfort or pain felt in the area between the chest and pelvis, often associated with gastrointestinal issues.
10. Rash: A rash is a noticeable change in the texture or color of the skin, which may be accompanied by itching, redness, or inflammation.
11. Oropharyngeal pain: Oropharyngeal pain refers to pain or discomfort felt in the back of the throat or the area around the tonsils.
It’s important to note that these adverse reactions were reported in clinical trials and may not necessarily occur in every patient. If any of these adverse reactions occur or persist, it is important to inform a healthcare professional for further evaluation and guidance. Additionally, there may be other less common or rare adverse reactions associated with Ngenla that are not listed here, so consulting the prescribing information and discussing any concerns with a healthcare provider is recommended.
Contraindications
Ngenla has several contraindications, meaning that its use is not recommended under certain conditions. The contraindications for Ngenla include:
1. Acute critical illness: Ngenla should not be used in patients who are experiencing acute critical illness. The medication is not indicated for the treatment of acute conditions.
2. Hypersensitivity: Ngenla should not be administered to patients who have a known hypersensitivity or allergic reaction to somatrogon-ghla (the active ingredient in Ngenla) or any of the excipients present in the formulation. Hypersensitivity reactions can range from mild to severe and can include symptoms such as rash, itching, swelling, or difficulty breathing.
3. Closed epiphyses: Ngenla is contraindicated in patients with closed epiphyses. Epiphyses are the ends of long bones that are responsible for bone growth. Once the epiphyses are closed, growth in height is no longer possible, and therefore, the use of Ngenla would not be beneficial.
4. Active malignancy: Ngenla should not be used in patients with active malignancy (cancer). The presence of an active malignancy is a contraindication due to potential risks associated with the use of growth hormone therapy in individuals with cancer.
5. Active proliferative or severe non-proliferative diabetic retinopathy: Ngenla is contraindicated in patients with active proliferative or severe non-proliferative diabetic retinopathy. These are eye conditions associated with diabetes that can worsen with the use of growth hormone therapy.
6. Prader-Willi syndrome with severe obesity or severe respiratory impairment: Ngenla is contraindicated in patients with Prader-Willi syndrome who are severely obese or have severe respiratory impairment. Prader-Willi syndrome is a genetic disorder characterized by insatiable hunger, and individuals with severe obesity or severe respiratory impairment may be at increased risk of complications with growth hormone therapy.
It’s important for healthcare providers to assess the patient’s medical history and consider these contraindications before prescribing Ngenla. If any of these contraindications are present, alternative treatment options or approaches may need to be considered.
Warnings
Ngenla has several important warnings and precautions associated with its use. These include:
1. Severe Hypersensitivity: Severe hypersensitivity reactions, although rare, can occur with Ngenla. If an allergic reaction is suspected, immediate medical attention should be sought.
2. Increased Risk of Neoplasms: Patients with preexisting tumors should be closely monitored for progression or recurrence. Childhood cancer survivors treated with growth hormone therapy, including Ngenla, may have an increased risk of developing a second neoplasm, particularly meningiomas in patients who received radiation to the head for their initial neoplasm.
3. Glucose Intolerance and Diabetes Mellitus: Ngenla may decrease insulin sensitivity, especially at higher doses. Regular monitoring of glucose levels is recommended for all patients receiving Ngenla, particularly those with existing diabetes mellitus or those at risk of developing it.
4. Intracranial Hypertension: Prior to initiating Ngenla treatment, fundoscopic examinations should be performed, and periodic evaluations thereafter. If preexisting papilledema (optic disc swelling) is identified, the underlying cause should be assessed and treated before starting Ngenla. If papilledema occurs during Ngenla treatment, treatment should be discontinued.
5. Fluid Retention: Fluid retention, which may manifest as edema or swelling, can occur with Ngenla and is dose-dependent. The dosage may need to be reduced if fluid retention becomes significant.
6. Hypoadrenalism: Patients with known hypoadrenalism should be monitored for reduced serum cortisol levels and the potential need for glucocorticoid dose adjustments.
7. Hypothyroidism: Thyroid function should be periodically monitored in patients receiving Ngenla, as hypothyroidism (underactive thyroid) may become apparent or worsen after initiating treatment.
8. Slipped Capital Femoral Epiphysis: Ngenla use has been associated with the development of slipped capital femoral epiphysis, a condition where the head of the thigh bone slips from the hip joint. Patients should be evaluated if they experience a limp or persistent hip or knee pain.
9. Progression of Preexisting Scoliosis: Monitoring for the development or progression of scoliosis (abnormal curvature of the spine) is recommended during Ngenla treatment.
10. Pancreatitis: Pancreatitis (inflammation of the pancreas) should be considered in patients experiencing persistent severe abdominal pain.
11. Lipoatrophy: Lipoatrophy, a loss of fat tissue, may occur if Ngenla is repeatedly injected into the same location over an extended period. Injection sites should be rotated to minimize this risk.
It is important for healthcare providers and patients to be aware of these warnings and precautions associated with Ngenla. Regular monitoring and appropriate management are necessary to mitigate potential risks and ensure patient safety.
Interactions
Ngenla may interact with other drugs, and it is important to consider these potential interactions. The following drug interactions have been identified:
1. Replacement Glucocorticoid Treatment: Patients receiving glucocorticoid therapy for hypoadrenalism may require an increase in their maintenance or stress dose following the initiation of Ngenla. Close monitoring and dose adjustments should be made in collaboration with a healthcare provider.
2. Pharmacologic Glucocorticoid Therapy and Supraphysiologic Glucocorticoid Treatment: Pediatric patients receiving pharmacologic glucocorticoid therapy should have their dosing adjusted to avoid both hypoadrenalism (insufficient adrenal gland function) and an inhibitory effect on growth. The healthcare provider will determine the appropriate glucocorticoid dosing regimen.
3. Cytochrome P450-Metabolized Drugs: Ngenla may potentially affect the clearance (metabolism) of drugs that are metabolized by the cytochrome P450 enzyme system. Close monitoring is recommended when these drugs are co-administered with Ngenla to ensure appropriate drug levels and efficacy.
4. Oral Estrogen: When Ngenla is used concurrently with oral estrogen therapy, larger doses of Ngenla may be required. The healthcare provider will assess and adjust the Ngenla dosage as necessary.
5. Insulin and/or Other Antihyperglycemic Agents: Ngenla may affect insulin sensitivity and glucose metabolism. Therefore, dose adjustments of insulin or other antihyperglycemic agents may be necessary when initiating or adjusting Ngenla therapy. Regular monitoring of blood glucose levels is important to ensure optimal glycemic control.
It is crucial for healthcare providers to be aware of these potential drug interactions and to consider them when prescribing Ngenla. Collaboration and communication between healthcare providers and patients are essential to ensure appropriate management and monitoring of any potential interactions.