Lead Poisoning Nightmare: Tainted Fruit Puree Pouches Cause 251 Cases Across 34 States
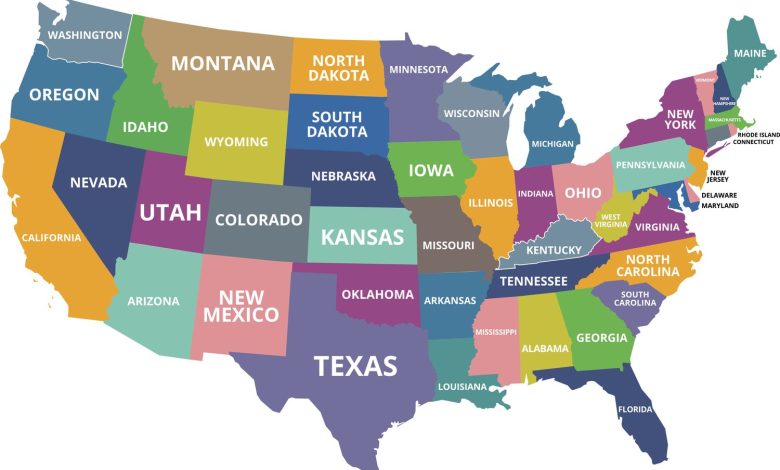
A startling surge in lead poisoning cases linked to contaminated fruit puree pouches has reached a staggering 251 reported cases across 34 states, according to recent updates from U.S. health officials. The alarming figure marks a significant increase from the previous weekly tally of 205 cases, as reported by the U.S. Centers for Disease Control and Prevention (CDC).
The tainted fruit puree pouches, now under recall, have been associated with AustroFoods’ WanaBana, Weis, and Schnucks brands of cinnamon-flavored applesauce. The CDC’s latest update reveals that affected states include Alabama, Arkansas, California, Colorado, Florida, Georgia, Iowa, Idaho, Illinois, Indiana, Kansas, Kentucky, Louisiana, Maine, Michigan, Minnesota, Missouri, Montana, North Carolina, North Dakota, Nebraska, New Hampshire, New Jersey, New York, Ohio, Oklahoma, Oregon, Pennsylvania, Tennessee, Texas, Virginia, Washington, Wisconsin, and West Virginia.
The CDC has highlighted the potential risks posed by lead poisoning, urging state health departments to actively seek out cases that may be missed if affected children do not undergo blood tests for lead exposure.
AustroFoods has announced plans to reimburse customers for lead tests, with a cap of up to $150. The Food and Drug Administration (FDA) recently reported alarming findings regarding cinnamon samples linked to the contaminated pouches, revealing toxic lead levels 2,000 times higher than proposed standards. Tests conducted at an Ecuadorian facility indicated lead contamination levels of 5110 parts per million (ppm) and 2270 ppm in cinnamon supplied by Negasmart, another company linked to Austrofoods.
The FDA is currently investigating the possibility of intentional contamination, with suspicions that the cinnamon used in the recalled applesauce may have been deliberately tainted with the toxic element. The recall involves three brands—Weis, WanaBana, and Schnucks—which can all be traced back to the same manufacturing facility in Ecuador.
Jim Jones, the FDA’s deputy commissioner for human foods, hinted at an intentional act within the supply chain. “We’re still in the midst of our investigation, but so far all of the signals we’re getting lead to an intentional act on the part of someone in the supply chain, and we’re trying to sort of figure that out,” he told Politico.
The FDA suspects the deliberate adulteration may have been economically motivated, and authorities from the U.S. and Ecuador are cooperating in the investigation. The FDA has limited authority over foreign ingredient suppliers not directly shipping products to the U.S., making it challenging to prevent intentional adulteration. However, officials are determined to identify those responsible and hold them accountable.
The recall advisory warns against consuming or serving the affected products and encourages families to discard the pouches or return them to the store for a refund. Caregivers are urged to have children who may have consumed the products undergo blood tests to check for lead exposure. Lead is particularly toxic to humans, especially children, and there is no safe level of exposure, with potential developmental delays and other severe health consequences.