Federal Judge Allows Lawsuit Over Ozempic Side Effects to Proceed
In a recent development, a federal judge in Louisiana has ruled against Novo Nordisk’s attempt to dismiss one of the earliest Ozempic lawsuits concerning the side effects of its widely-used drug, Ozempic. U.S. District Judge James Cain Jr, in his decision on Friday, largely rejected Novo Nordisk’s bid to dismiss the case, allowing plaintiff Jaclyn Bjorklund’s claims to move forward.
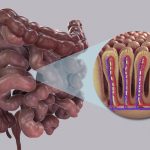
The lawsuit alleges that Novo Nordisk failed to adequately warn doctors about the risk of gastroparesis, a condition characterized by a slowdown in the emptying of the stomach into the small intestine, associated with Ozempic. While the judge dismissed Bjorklund’s breach of express warranty claim, stating that she did not identify specific promises made by Novo Nordisk, he allowed her to amend and refile the claim, leaving the door open for further legal proceedings.
Ozempic, a semaglutide injection drug used for treating type 2 diabetes, has been a blockbuster drug for Novo Nordisk. However, concerns over potential side effects have led to legal challenges. The plaintiff, Jaclyn Bjorklund, alleges that her severe vomiting and pain, which ultimately led her to the hospital and caused her to lose teeth, were a result of taking Ozempic for over a year before switching to Mounjaro, a drug made by Eli Lilly, in July 2023.
Novo Nordisk has argued that the bad side effects of Ozempic in question are well known and documented in the drug’s U.S. Food and Drug Administration (FDA)-approved label. The company believes that the allegations in the lawsuit are without merit and has expressed its intention to vigorously defend against the claims. Novo Nordisk emphasizes that its drugs, including Ozempic, have undergone extensive studies, and their safety is continually monitored.
On the other hand, Eli Lilly, the manufacturer of Mounjaro, stated that Bjorklund’s lawsuit is without merit. The company contends that it diligently monitors, evaluates, and reports safety information for all its medicines. Eli Lilly has filed a separate motion to dismiss, and the outcome of that motion is still pending, according to court records.
This ruling comes amid an ongoing debate about the transparency of pharmaceutical companies in disclosing potential side effects and the adequacy of warnings provided to both healthcare professionals and patients. The case could set a precedent for similar lawsuits involving the alleged failure to warn about the risks associated with specific medications. As the legal proceedings continue, the pharmaceutical industry and regulatory bodies will likely face increased scrutiny regarding the communication of potential side effects of prescription drugs.