FDA Issues Warning and Labeling Updates for Promethazine Hydrochloride Injection Products
In an announcement targeted at Health Care Professionals, the U.S. Food and Drug Administration (FDA) has released information regarding labeling updates for products containing Promethazine Hydrochloride Injection. The primary objective of these updates is to further mitigate the risk of severe chemical irritation and tissue damage associated with intravenous administration of this medication.
Promethazine hydrochloride injection is commonly used for managing allergic reactions, motion sickness, post-operative nausea and vomiting, and as a sedative or adjunct to analgesics.
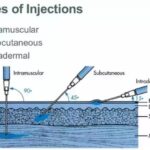
The FDA strongly advises health care professionals to administer Promethazine Hydrochloride Injection through deep intramuscular administration as opposed to intravenous administration. If intravenous administration is deemed necessary, health care professionals are urged to carefully review and adhere to the updated information provided in the labeling. This includes specific guidelines on dilution and intravenous infusion to minimize the risk of severe tissue injury.
Key recommendations and updates include:
1. Deep Intramuscular Administration Preference:
• Health care professionals are encouraged to administer Promethazine Hydrochloride Injection through deep intramuscular administration, favoring this method over intravenous administration.
2. Intravenous Administration Guidelines:
• In cases where intravenous administration is unavoidable, health care professionals must dilute Promethazine Hydrochloride Injection as recommended.
• The FDA emphasizes that intravenous administration should be through an intravenous catheter inserted in a large vein, preferably via a central venous catheter. Intravenous catheters in the hand or wrist should be avoided.
• Promethazine Hydrochloride Injection should not be mixed with other drugs or diluted with solutions other than 0.9% sodium chloride injection.
• Intravenous injection at concentrations greater than 1 mg/mL is contraindicated.
3. Preparation and Infusion Instructions:
• Specific preparation and infusion information for both adult and pediatric patients are provided, detailing the volume of 0.9% Sodium Chloride Injection for dilution, maximum concentration of the diluted solution, and maximum rate of infusion.
The FDA has mandated manufacturers to update prescribing information, carton labeling, and container labels with this new safety information. Health care professionals, caregivers, and patients are encouraged to report any adverse events or side effects related to the use of Promethazine Hydrochloride Injection to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
For reporting adverse events, individuals can use the MedWatch Online Voluntary Reporting Form on the FDA’s official website or download the form and submit it via fax at 1-800-FDA-0178.