Cabometyx Patent Battle Resolved As Exelixis Grants Teva 2031 Generic License in Settlement
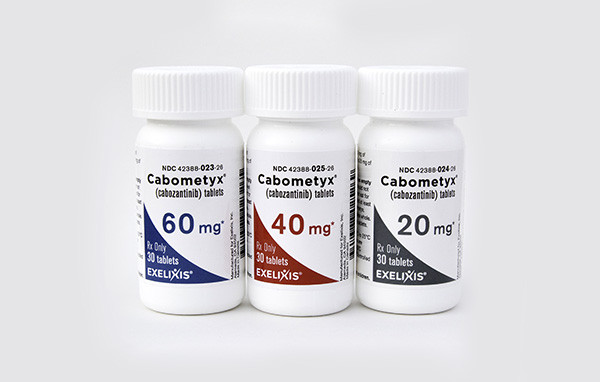
In recent years, Exelixis has been involved in a fierce battle to safeguard its flagship cancer drug, Cabometyx, by defending its intellectual property. In a recent development, Exelixis has reached a patent agreement with the generics giant, Teva, adding another layer of defense to protect its valuable drug.
The patent litigation arose when Teva submitted a generic application to the FDA. In response, Exelixis filed a patent lawsuit claiming infringement of its patent No. 11,298,349, which is set to expire in February 2032. Earlier, in 2021, Exelixis had also filed a lawsuit related to three other patents, with the latest one scheduled to expire in July 2033.
After a period of legal wrangling, Exelixis and Teva have now settled their patent disputes in the Delaware federal court. As part of the settlement, Exelixis has agreed to grant Teva a license to market its generic version of Cabometyx from January 1, 2031, provided it receives FDA approval for the generic product.
The financial details of the settlement remain undisclosed. The agreement is currently pending review by the U.S. authorities, and both companies are expected to comply with the terms once the regulatory approval is obtained.
Apart from its legal tussle with Teva, Exelixis is also pursuing patent claims against MSN Labs in India. In a previous ruling, the Delaware federal court ruled in favor of Exelixis, rejecting MSN’s challenge to patent No. 7,579,473. Following this favorable judgment, Exelixis intends to seek an order from the court, preventing regulatory approval of MSN’s generic version until August 2026.
Despite the legal battles, Cabometyx has been a commercial success for Exelixis since its initial approval in April 2016 for the treatment of specific patients with renal cell carcinoma. The drug’s effectiveness also led to approvals for treating hepatocellular carcinoma and thyroid cancer, contributing significantly to Exelixis’ revenue. In the previous year, the Cabometyx franchise generated an impressive $1.4 billion in sales.
However, amidst the positive commercial performance, there have been some clinical setbacks for Cabometyx in combination with Roche’s Tecentriq. In a recent trial, the combination therapy failed to show improved outcomes compared to Cabometyx alone in kidney cancer patients who had progressed on initial immunotherapy. Similar disappointing results were observed in trials for liver cancer and lung cancer treatments.
Despite these challenges, Exelixis remains committed to protecting its valuable drug and continues to explore new opportunities and partnerships to advance cancer treatment and improve patient outcomes. As the pharmaceutical landscape evolves, Exelixis seeks to maintain its position as a key player in the oncology field and bring innovative therapies to patients in need.