Why Do-It-Yourself Medications Are on the Rise
Written by Kathleen Doheny
“I’ve always been a little wary of needles,” Heather, 65, a resident of Southern California, said as she reminisced about a long-ago high school biology class. The instructor asked them all to prick their finger to find out their blood type. It took her the whole hour to work up her nerve, said Heather, who asked that her real name not be used to protect her privacy, but she did it.
Several decades later, the challenge surfaced again. Her doctor decided to add the lowest dose of Ozempic (semaglutide), injected once a week, to her dose of oral metformin to help manage her blood sugar.
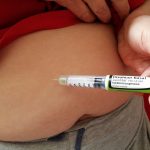
“It’s a tiny little needle, and it’s an automatic injector,” Heather told herself, yet she felt like she was right back in high school biology class. So her husband did the honors for the first dose. It wasn’t nearly as bad as she imagined, she said. The needle, she said, was short and fine.
“I felt the medicine going in a little bit and some stinging. The next week, I did it on my own,” she said.
Heather’s off the Ozempic now, her blood sugar managed well again just with metformin. But she, as well as the rest of us, should expect to be taking more injectables in the future, experts say. The era of do-it-yourself medicine, via self-injection at home, is here, growing, and shows no signs of slowing down.
In the past, self-injected medicine was mainly insulin, injected by those with diabetes, along with anti-coagulants for those at high risk of blood clots, said Eric J. Topol, MD, editor-in-chief of Medscape (WebMD’s sister publication for health care professionals), a professor of molecular medicine, and executive vice president of Scripps Research in La Jolla, CA.
“Fast forward,” Topol said. “Now we have all these autoimmune disease drugs [that can be self-injected]. We have these anti-obesity, anti-diabetes drugs, we have the powerful low-cholesterol agents, the drugs like Repatha [evolocumab]. We have people taking two or three different injectable drugs every other week.”
All this, he said, comes after many people, just 2 years ago, claimed they had “needle phobia” when offered a COVID-19 vaccine. In one U.K. study, researchers who polled more than 15,000 adults and matched them to a general population sample concluded that about 10% of vaccine hesitancy was due to fear of blood, needles, or invasive medical procedures.
“And now we are in a world where we are training the public to inject themselves,” Topol said.
The market for self-injected drugs is increasing, with no signs of slowing down, according to analysts’ reports. While estimates range greatly, one analysis estimated that the global self-injection devices market size was $6.6 billion in 2021 and would grow nearly 6% a year from 2022 to 2030.
Self-injected devices include prefilled syringes or pens and auto-injectors. As of August 2021, according to a market review, nearly 80 auto-injectors have been developed by more than 20 drug companies. When researchers evaluated 2,964 shots given from the auto-injectors, just 12 device malfunctions occurred, for a failure rate of 0.40%.
Chances are, someone you know self-injects a medication, such as Humira (adalimumab) for arthritis, Repatha (evolocumab) to manage cholesterol, Dupixent (dupilumab) for asthma or, yes, Ozempic (semaglutide) for diabetes control or Wegovy (semaglutide) for weight loss.
Three key things are driving this trend, according to George I’ons, head of product strategy for Owen Mumford Ltd. in Oxford, U.K., which designs, develops, and makes injectable drug delivery systems for drug, biotech, and generics industries. These include:
- Staff shortages at medical clinics and hospitals
- Financial pressures on health care systems
- A growing aging population, likely to need more medications on a regular basis
Having patients give themselves shots, when possible, not only saves clinic time and expense, but also spares the patients a trip to the clinic, of course, and often a copay. “The more people can do for themselves, the less you need to occupy staff time,” I’ons said. That means more time staff can spend on areas that really need attention. That 20 or 30 minutes of clinic time that don’t need to be spent giving someone medicine can be put to valuable use, he said.
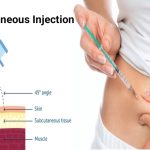
Improved Devices, Needles
While the needle phobics may shudder at the self-injector trend, ongoing device improvements are aimed at comfort. For instance, I’ons said, “a lot of auto-injectors hide the needle before and after use.” The user feels just a piece of plastic against the skin.
Now we are in a world where we are training the public to inject themselves. Needles have often gotten so thin, that at least with some devices, “you could quite easily not even feel the thing going in,” he said. For insulin delivery, I’ons said, the needles have gotten thinner and shorter over the years.
But not all medicines delivered by self-injector can use the smallest needle, he said. Some drugs, because of their viscosity, or thickness, may need bigger needles.
When people complain of discomfort as the medication is injected, they could be feeling not the tiny needle, but the drug itself, or one of its inactive ingredients, I’ons said. He cited the case of drug maker AbbVie removing the buffer, citrate, from Humira (adalimumab) and offering a citrate-free version in 2018 because the citrate was linked to pain where people got the shot.
Companies said they are focused on technology advances to make self-injection less unpleasant. “Significant advances in technology, as well as our investments in R&D, have allowed Lilly to explore a variety of different injection methods over the years,” Nadia Ahmad, MD, associate vice president and medical director of obesity clinical development for Eli Lilly & Company, said in a statement.
Some people prefer the shots over pills, she said, because “in some cases, it may lead to higher adherence and better efficacy through consistent use.”
At Amgen, an executive said demand is growing from patients and providers “to have flexibility when it comes to administration of our medicines.” Jyothis George, vice president and global medical therapeutic area head in general medicine at Amgen, said in a statement that for Repatha, for example, grew 32% in the first quarter of this year. In February, the FDA approved self-administration of Tezspire (Tezepelumab-ekko), developed by Amgen and AstraZeneca, for patients with severe asthma.
Education Helps Self-Injection Skills
Introducing patients to self-injections is part of the day’s work for Amy Hess-Fischl, a certified diabetes educator and registered dietitian who works as a diabetes educator at the University of Chicago. “As they sit down, I hand them a needle, a syringe, and say, ‘Go ahead and inject.’ Once they do that, they say, ‘Oh my God, it’s so small.’” It gets the anxiety of the unknown out of the way, she said.
She’s talking about insulin injections. “When it comes to these other injectables, with so many, you don’t even see the needle.”
She reminds patients that the needles are decreasing in size, in general, in both the length and the thickness, or gauge. Some needles are now so short and so small, they can be compared to an eyelash, she said.
She reminds patients, too, that self-injecting can be empowering. “It’s about patient-centered care. I think this new revolution in injectables is going to improve patient-centered care and reduce anxiety.”
Support and education are important, she said. While there are online resources for self-injecting, the human touch remains important, she said. Any health care providers prescribing a self-injected medication, Hess-Fischl said, “needs to have a plan about where to send this patient to be successful.” If the health care provider doesn’t come through, she suggests patients call the drugmaker, and staff there should be able to give instruction, or tell them where to get the instruction.
The Next Market?
While many drugs can be self-injected, not all can. Many patients with chronic diseases depend on drugs that must be delivered with an IV, which means spending hours in a clinic or other facility.
One barrier is the high viscosity of some of these medicines, making it impossible to make and inject some of the monoclonal antibodies at the small volumes needed for shots under the skin.
Science could change that, said Jeffrey Hackman, CEO and chairman of Comera Life Sciences, who has come up with ways to change some biologics into forms that could be given under the skin and self-injected by the patient at home.
“I don’t think we can ever get out of IV medicines [entirely],” he said. But he has hopes that some biologics now given via IV at clinics will be self-injected at home within the next 5 to 7 years, and much more quickly than the process now requires.