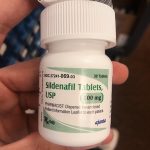
WEFUN Inc. Issues Voluntary Nationwide Recall of WEFUN Capsules Due to Presence of Undeclared Sildenafil

Brooklyn, New York, September 5, 2023 – WEFUN Inc., a dietary supplement manufacturer, has initiated a voluntary nationwide recall of 300 boxes of WEFUN Capsules due to the presence of undeclared sildenafil, an ingredient commonly used in FDA-approved products for the treatment of male erectile dysfunction. The Food and Drug Administration (FDA) conducted an analysis that revealed the contamination, prompting this recall. This development poses potential health risks to consumers, particularly those who may be using nitrates for various medical conditions.
Risk Statement: Consuming products that contain undisclosed sildenafil can be dangerous, especially when it interacts with nitrates found in some prescription medications like nitroglycerin. This interaction can result in a significant drop in blood pressure, which could be life-threatening. Individuals with diabetes, high blood pressure, high cholesterol, or heart disease are often prescribed nitrates, making them more susceptible to these risks. It’s important to note that WEFUN Inc. has not received any reports of adverse events related to this recall at this time.
Identification of Affected Product: The affected product, WEFUN Capsules, is marketed as a dietary supplement and is packaged in blue cardboard boxes, each containing 10 capsules. The specific lot numbers subject to this recall are #18520168 and 09/30/2026. These capsules were distributed nationwide in the USA through online sales via Amazon.com and eshoponlineusa.com.
Recall Procedure: WEFUN Inc. is actively notifying its distributors and customers via email and coordinating the return of all recalled WEFUN capsules. Consumers, distributors, and retailers who possess the affected WEFUN capsules should discontinue use and return the product to the place of purchase.
Consumer Contact Information: For any questions or concerns related to this recall, consumers can reach out to WEFUN Inc. by phone at 929-509-7343. The helpline is available seven days a week from 9 am to 7 pm Eastern Time. Alternatively, inquiries can be sent via email to wefun2022@gmail.com. In cases where consumers have experienced problems possibly linked to this product, it is advisable to contact their physician or healthcare provider.
Reporting Adverse Reactions: Adverse reactions or quality issues associated with the use of this product can be reported to the FDA through the MedWatch Adverse Event Reporting program. Reports can be submitted online, by regular mail, or by fax, with contact information available at the FDA’s website.
This recall is being conducted in full cooperation with the U.S. Food and Drug Administration to ensure the safety and well-being of consumers.
Source: FDA
For more information and updates on this recall, please refer to additional news resources or visit the FDA’s official website.