Why Makena Was Withdrawn From the U.S. Market

The World Health Organization defines preterm delivery as babies born alive before 37 weeks of pregnancy are completed. There are sub-categories of preterm birth, based on gestational age:
- extremely preterm (less than 28 weeks)
- very preterm (28 to 32 weeks)
- moderate to late preterm (32 to 37 weeks).
Babies may be born preterm because of spontaneous preterm labor or because there is a medical indication to plan an induction of labor or cesarean birth early.
An estimated 15 million babies are born too early every year. That is more than 1 in 10 babies. Approximately 1 million children die each year due to complications of preterm birth . Many survivors face a lifetime of disability, including learning disabilities and visual and hearing problems.
What is Makena?
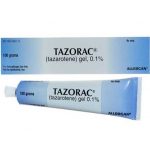
Makena is a progestin-based medication supplied by Covis Pharma. it is used to reduce the risk of preterm birth in women with a singleton pregnancy who have a history of singleton spontaneous preterm birth. The effectiveness of Makena was based on improvement in the proportion of women who delivered <37 weeks of gestation. It is the only FDA-approved drug to prevent preterm births.
Why Makena Was Withdrawn From the U.S. Market?
Makena was withdrawn from the U.S. market after the US Food and Administration panel said it is not effective at preventing preterm births. This is coming months after a panel voted 14-1 to retract the treatment, according to The Wall Street Journal. In October 2022, an FDA advisory committee met for two and a half days to discuss the drug’s efficacy after a 2019 trial of 1,000 women found the drug did not reduce the risk of a preterm birth. All 15 members agreed the trial showed Makena had no clinical benefit, and nearly all recommended that the FDA yank its approval.
Before the FDA could issue a decision, Covis Pharma said March 7 it wants to work with the agency to enact a wind-down period so patients who take the drug can adjust.
“While we stand by Makena’s favorable benefit-risk profile, including its efficacy in women at highest risk of preterm birth, we are seeking to voluntarily withdraw the product and work with the FDA to effectuate an orderly wind-down,” Covis Chief Innovation Officer Raghav Chari, PhD, said.
If the FDA agrees, people who currently take Makena could finish their 21-week treatment course, according to the Journal.
Ways of Preventing or Reducing the Risk of Premature Birth
Live a healthy lifestyle
• Avoid tobacco, smoking, e-cigarettes, and secondhand smoke
• Don’t drink alcohol while trying to get pregnant and during pregnancy
• Don’t use street drugs and avoid misuse of prescription drugs
• Eat a balanced diet with foods containing iron and folic acid
• Be active every day: try to get 30 minutes of exercise every day
• Get medical conditions like diabetes and high blood pressure under control
• Lose weight to avoid being obese; if you are underweight, gain weight
• Lower your stress levels: try yoga, meditation, being active, support groups, balance work, and your life
• Work on having a healthy relationship with your partner without violence
Take good care of yourself and your baby during pregnancy
• Seek prenatal care early in your pregnancy, particularly if you have any risk factors for preterm birth such as having had a premature baby in the past, or having a problem with your uterus or cervix
• Attend prenatal visits with your partner
• Tell your physician or midwife if you think you are having signs of premature labor (below)
• Live a healthy lifestyle
• If you and your baby are healthy, it is best to wait until at least 39 weeks and let labor begin on its own