TL 177 Pill: Uses, Side Effects, Abuse, Warnings
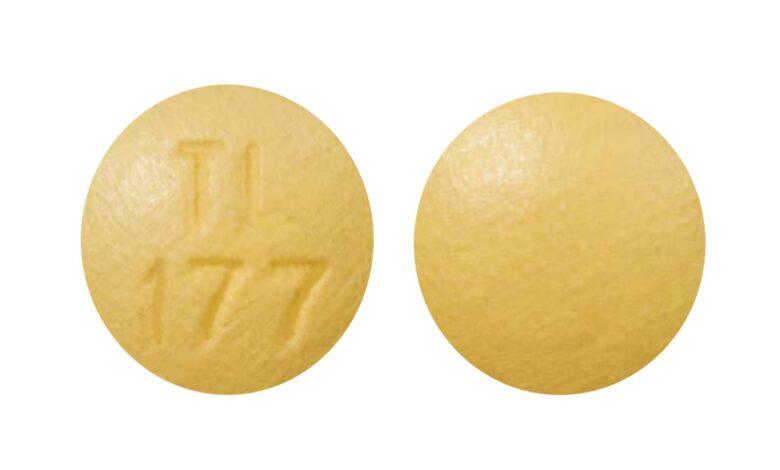
The yellow round pill with the imprint TL 177 has been identified as Cyclobenzaprine Hydrochloride 10 mg. It is supplied by Breckenridge Pharmaceutical, Inc.. Cyclobenzaprine belongs to the family of medications known as muscle relaxants. Cyclobenzaprine is not currently controlled under the Controlled Substances Act.
TL 177 pill is used along with rest and physical therapy for the relief of muscle spasm associated with acute (sudden and short-term) painful conditions. Muscle relaxants work in the central nervous system (CNS) to modify signals from the brain that cause the muscles to tighten. When used in combination with rest and physical therapy, muscle relaxants help to relieve the pain and stiffness caused by muscle spasm.
How should I use TL 177 pill?
The usual adult dose of TL 177 pill (cyclobenzaprine) is 10 mg 3 times a day, with a range of 20 mg to 40 mg a day (given in divided doses). The total dose should not exceed 60 mg daily. Use of this medication is not recommended for periods longer than 2 or 3 weeks.
Many things can affect the dose of medication that a person needs, such as body weight, other medical conditions, and other medications. If your doctor has recommended a dose different from the ones listed here, do not change the way that you are taking the medication without consulting your doctor.
It is important to take this medication exactly as prescribed by your doctor. If you miss a dose, take it as soon as possible and continue with your regular schedule. If it is almost time for your next dose, skip the missed dose and continue with your regular dosing schedule. Do not take a double dose to make up for a missed one. If you are not sure what to do after missing a dose, contact your doctor or pharmacist for advice.
What happens if you take a lot of TL 177 pill?
The most common effects associated with TL 177 pill overdose are drowsiness and tachycardia. Less frequent manifestations include tremor, agitation, coma, ataxia, hypertension, slurred speech, confusion, dizziness, nausea, vomiting, and hallucinations.
When abused, TL 177 pill may have a sedative and relaxing effect and potentially even cause a euphoric “high.” TL 177 pill may be abused orally, mixed with other drugs, easily dissolved in alcohol, or crushed to be snorted.
Who should NOT take TL 177 pill?
Do not take this medication if you:
• are allergic to cyclobenzaprine or any ingredients of this medication
• are in the acute recovery phase of heart attack
• are taking MAO inhibitors (such as phenelzine or tranylcypromine) or have taken them in the past 14 days
• have abnormal heart rhythms
• have an overactive thyroid gland (hyperthyroidism)
• have congestive heart failure
• have heart block or conduction disturbances
What are the side effects of TL 177 pill?
The following side effects have been reported by at least 1% of people taking TL 177 pill. Many of these side effects can be managed, and some may go away on their own over time.
Contact your doctor if you experience these side effects and they are severe or bothersome. Your pharmacist may be able to advise you on managing side effects.
• blurred vision
• difficulty sleeping
• dizziness
• drowsiness
• dry mouth
• fatigue
• headache
• indigestion
• nausea
• sweating
• unpleasant taste
• weakness
Although most of the side effects listed below don’t happen very often, they could lead to serious problems if you do not seek medical attention.
Check with your doctor as soon as possible if any of the following side effects occur:
• abdominal pain
• agitation
• confusion
• constipation
• difficulty urinating
• hallucinations (seeing or hearing things that aren’t there)
• increased heart rate or fast heartbeat
• lack of muscle coordination or clumsiness
• sensations of tingling, burning, or numbness
• shortness of breath
• signs of depression (e.g., poor concentration, changes in weight, changes in sleep, decreased interest in activities, thoughts of suicide)
• symptoms of glaucoma (e.g., blurred vision, seeing halos of bright colours around lights, red eyes, increased pressure in your eyes, eye pain or discomfort)
• trembling
Stop taking the medication and seek immediate medical attention if any of the following occur:
• fainting
• symptoms of overdose
o dry, hot, flushed skin
o fast or irregular heartbeat
o hallucinations
o increase or decrease in body temperature
o seizures
o severe drowsiness
o troubled breathing
o unexplained muscle stiffness
o vomiting occurring together with other symptoms of overdose
• symptoms of serious allergic reaction
o changes in the skin colour of the face
o fast or irregular breathing
o large swellings that look like hives on the face, eyelids, mouth, lips, and/or tongue
o puffiness or swelling of the eyelids or the area around the eyes
o shortness of breath
o skin rash, hives, or itching
o tightness in chest
o trouble breathing
o wheezing
• symptoms of serotonin syndrome
o confusion
o fast heartbeat
o hallucinations
o restlessness
o shaking
o shivering
o sudden jerking of muscles
o sweating
Some people may experience side effects other than those listed. Check with your doctor if you notice any symptom that worries you while you are taking this medication.
Are there any other precautions or warnings for this medication?
Before you begin using a medication, be sure to inform your doctor of any medical conditions or allergies you may have, any medications you are taking, whether you are pregnant or breast-feeding, and any other significant facts about your health. These factors may affect how you should use this medication.
Drowsiness/reduced alertness: Cyclobenzaprine may impair the ability to perform hazardous tasks such as operating machinery or driving a vehicle. Do not drive or engage in other activities requiring alertness until you determine how the medication affects you.
Glaucoma: TL 177 pill may cause increased pressure in the eye, triggering symptoms of glaucoma. If you have glaucoma or elevated pressure in your eye(s), discuss with your doctor how this medication may affect your medical condition, how your medical condition may affect the dosing and effectiveness of this medication, and whether any special monitoring is needed.
Length of treatment: The use of TL 177 pill for periods longer than 2 or 3 weeks is not recommended.
Liver function: Liver disease or reduced liver function may cause this medication to build up in the body, causing side effects. If you have liver problems, discuss with your doctor how this medication may affect your medical condition, how your medical condition may affect the dosing and effectiveness of this medication, and whether any special monitoring is needed.
Serotonin syndrome: Severe reactions are possible when TL 177 pill is combined with other medications that act on serotonin, such as certain antidepressants and certain medications for migraine headache. Symptoms of a reaction may include muscle rigidity and spasms, difficulty moving, changes in mental state including delirium and agitation. Coma and death are possible.
If you are taking other medications, discuss with your doctor how these medications may affect your medical condition, how your medical condition may affect the dosing and effectiveness of these medications, and whether any special monitoring is needed.
Urinary problems: TL 177 pill can make urination difficult. People who have difficulty starting urine flow may find that this becomes worse while taking this medication. If you have a history of urinary retention, discuss with your doctor how this medication may affect your medical condition, how your medical condition may affect the dosing and effectiveness of this medication, and whether any special monitoring is needed.
Pregnancy: This medication should not be used during pregnancy unless the benefits outweigh the risks. If you become pregnant while taking TL 177 pill, contact your doctor immediately.
Breast-feeding: This medication may pass into breast milk. If you are a breast-feeding mother and are taking TL 177 pill, it may affect your baby. Talk to your doctor about whether you should continue breast-feeding.
Children: The safety and effectiveness of TL 177 pill for children below the age of 15 have not been established.
What other drugs could interact with this medication?
There may be an interaction between cyclobenzaprine and any of the following:
• abiraterone acetate
• aclidinium
• amlodipine
• amphetamines (e.g., dextroamphetamine, lisdexamphetamine)
• antihistamines (e.g,. cetirizine, doxylamine, diphenhydramine, hydroxyzine, loratadine)
• antipsychotics (e.g., chlorpromazine, clozapine, haloperidol, olanzapine, quetiapine, risperidone)
• atropine
• azelastine
• belladonna
• benzodiazepines (e.g., alprazolam, diazepam, lorazepam)
• benztropine
• botulinum toxin
• brimonidine
• bromocriptine
• buspirone
• cannabis
• certain quinolone antibiotics (e.g., ciprofloxacin, norfloxacin, ofloxacin)
• cimetidine
• darifenacin
• deferasirox
• dextromethorphan
• disopyramide
• domperidone
• entacapone
• ergot alkaloids (e.g., ergotamine, dihydroergotamine)
• ethinyl estradiol
• fesoterodine
• flavoxate
• gemfibrozil
• glucagon
• glycopyrrolate
• ipratropium
• kava kava
• ketoconazole
• lithium
• magnesium sulphate
• methylene blue
• methoxsalen
• metoclopramide
• metyrosine
• mexiletine
• mianserin
• minocycline
• mirabegron
• mirtazapine
• monoamine oxidase inhibitors (MAOIs; e.g., moclobemide, rasagiline, selegiline, tranylcypromine)
• narcotic pain relievers (e.g., codeine, fentanyl, morphine, oxycodone)
• nabilone
• nifedipine
• orphenadrine
• oxybutynin
• peginterferon alfa-2b
• potassium chloride
• pramipexole
• pramlintide
• primaquine
• rivastigmine
• ropinirole
• rotigotine
• scopolamine
• seizure medications (e.g., carbamazepine, clobazam, levetiracetam, phenobarbital, phenytoin, primidone, topiramate, valproic acid)
• serotonin antagonists (anti-emetic medications; e.g., granisetron, ondansetron)
• serotonin/norepinephrine reuptake inhibitors (SNRIs; e.g., desvenlafaxine, duloxetine, venlafaxine)
• selective serotonin reuptake inhibitors (SSRIs; e.g., citalopram, fluoxetine, paroxetine, sertraline)
• solifenacin
• thalidomide
• thiazide diuretics (water pills; e.g., hydrochlorothiazide, indapamide, metolazone)
• tiotropium
• tizanidine
• tolcapone
• tolterodine
• tramadol
• trazodone
• tricyclic antidepressants (e.g., amitriptyline, doxepin, imipramine, nortriptyline)
• triptan medications (e.g., eletriptan, frovatriptan, rizatriptan, sumatriptan)
• tryptophan
• umeclidinium
• vemurafenib
• verapamil
• zolpidem
• zopiclone
If you are taking any of these medications, speak with your doctor or pharmacist. Depending on your specific circumstances, your doctor may want you to:
• stop taking one of the medications,
• change one of the medications to another,
• change how you are taking one or both of the medications, or
• leave everything as is.
An interaction between two medications does not always mean that you must stop taking one of them. Speak to your doctor about how any drug interactions are being managed or should be managed.
Medications other than those listed above may interact with this medication. Tell your doctor or prescriber about all prescription, over-the-counter (non-prescription), and herbal medications you are taking. Also tell them about any supplements you take. Since caffeine, alcohol, the nicotine from cigarettes, or street drugs can affect the action of many medications, you should let your prescriber know if you use them.