Canex V Cream: Uses, Dosage, Side Effect, Interactions
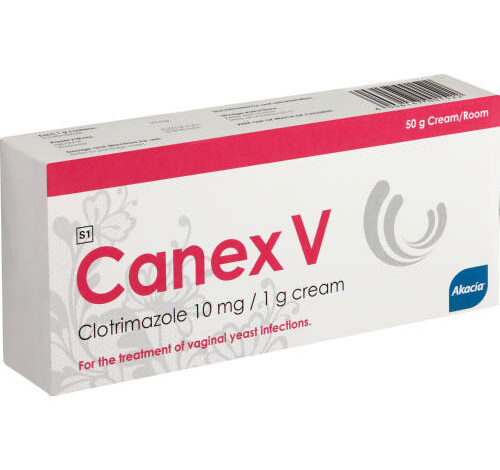
Canex V cream is a brand of clotrimazole for use in the vagina. Clotrimazole is in a class of antifungal medications called imidazoles. It works by stopping the growth of fungi that cause infection. Canex V cream is used to treat vaginal yeast infections in adults and children 12 years of age and older.
Most yeast infections are caused by a type of fungus (candida) called Candida albicans. Other kinds of fungus can cause yeast infections too, but antifungal treatments usually only target the most common one. If your infection is caused by something different, Canex V cream may not be effective for you.
Around 75% of women will get at least one yeast infection in their lifetime, up to 8% get more than four a year. They’re called recurrent yeast infections when they happen over and over.
How to Apply Canex V Cream
To apply Canex vaginal cream, you will need a towel, soap, and water.
Prepare
Find a comfortable place where you can lie down while applying the cream. Your bed can be an ideal option, though you may want to place a towel underneath you to prevent any cream from spilling on your linens.
Steps
- Wash your hands with soap and water.
- Open the tube.
- Screw the applicator nozzle onto the tube until it is secure but not overly tight.
- Gently squeeze the tube from the bottom to push a sufficient amount of cream into the applicator barrel. Make sure it is enough to reach the prescribed dose. Most applicators provide markings to indicate where you should stop.
- Unscrew the applicator from the tube.
- Lie on your back with your knees drawn toward you.
- Gently insert the applicator deep into your vagina.
- Press the plunger down until it reaches its original position.
- Wash your hands with soap and water after applying the cream.
Note, if you are pregnant, insert the applicator gently and don’t insert it past the point where you feel resistance.
Cleaning the Applicator
Reusable applicators should be cleaned by pulling the plunger to remove it from the barrel and washing it with mild soap and warm water. Wipe it dry and allow it to air dry while disassembled. You can assemble it to store away once it is dry, such as in the morning if you are using it before bedtime. Never boil your reusable applicator or use extremely hot water, as this can cause the plastic to melt or deteriorate.
If you are using Canex V cream to treat an infection, you should discard the applicator once you have finished your course of treatment. The used applicator could transfer yeast, bacteria, and other microorganisms if you were to reuse it in the future.
What are the side effects of Canex V Cream?
Canex V cream may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- increased burning, itching, or irritation of the vagina
Some side effects can be serious. If you experience any of the following symptoms, stop using Canex V Cream and call your doctor immediately:
- rash
- hives
- stomach pain
- fever
- chills
- nausea
- vomiting
- foul-smelling vaginal discharge
If you experience a serious side effect, you or your doctor may send a report to the Food and Drug Administration’s (FDA) MedWatch Adverse Event Reporting program online (http://www.fda.gov/Safety/MedWatch) or by phone (1-800-332-1088).
What should I watch out for while using this medicine?
Tell your doctor or health care professional if your symptoms do not start to get better within a few days.
It is better not to have sex until you have finished your Canex V cream treatment. Canex V cream may damage condoms or diaphragms and cause them not to work properly. It may also decrease the effect of vaginal spermicides. Do not rely on Canex V cream to prevent sexually transmitted diseases or pregnancy while you are using this medicine.
Vaginal medicines like Canex V cream usually come out of the vagina during treatment. To keep the medicine from getting on your clothing, wear a mini-pad or sanitary napkin. The use of tampons is not recommended since they may soak up the medicine. To help clear up the infection, wear freshly washed cotton, not synthetic, underwear.
Which medications can interact with Canex V Cream?
The effects of Canex V cream can change if you take other drugs or herbal products at the same time. This can increase your risk for serious side effects or may cause your medications not to work correctly. These drug interactions are possible, but do not always occur. Your doctor or pharmacist can often prevent or manage interactions by changing how you use your medications or by close monitoring.
To help your doctor and pharmacist give you the best care, be sure to tell your doctor and pharmacist about all the products you use (including prescription drugs, nonprescription drugs, and herbal products) before starting treatment with this product. While using this product, do not start, stop, or change the dosage of any other medicines you are using without your doctor’s approval.
Some products that may increase the risk of vaginal yeast infections include antibiotics, corticosteroids (such as prednisone), and drugs that suppress the immune system (such as cyclosporine, methotrexate).
Keep a list of all the products you use. Share the list with your doctor and pharmacist to reduce your risk for serious medication problems. Also tell them if you smoke, drink alcohol, or use illegal drugs. Some items may interact with your medicine. You can also find useful information on: Home Remedies For Yeast Infection That Work