Novartis Issues Voluntary US Nationwide Recall of Two Lots of Sandimmune® Oral Solution (cyclosporine oral solution, USP), 100 mg/mL due to Crystallization
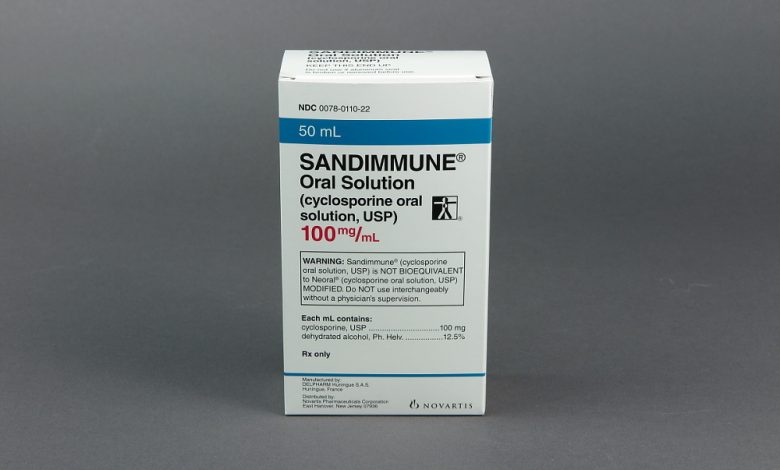
Novartis Pharmaceuticals Corporation has initiated a voluntary nationwide recall of two lots of its Sandimmune® Oral Solution (cyclosporine oral solution, USP), 100 mg/mL in the US. The recall, prompted by the observation of crystal formation in some bottles, aims to prevent potential incorrect dosing.
The affected lots, distributed between January 2022 and September 2022, were identified as FX001500 (expiration date 09/2024) and FX001582 (expiration date 09/2024), with National Drug Code (NDC) 0078-0110-22.
Nature of the Recall: Crystal Formation and Risk Implications
The decision to recall was made following an investigation into crystallization in a different lot of Sandimmune® Oral Solution (cyclosporine oral solution, USP), 100 mg/mL. The crystallization of cyclosporine in the oral solution may lead to non-uniform distribution, posing a risk of under-dosing or over-dosing. Under-dosing could result in lower exposure, decreased efficacy, and potential graft rejection and loss in transplant patients. Conversely, over-dosing may lead to cyclosporine toxicity in the long term. Novartis has not received any adverse event reports related to this recall to date.
Product Details and Indications
Sandimmune® Oral Solution, 100 mg/mL, is packaged in 50 mL bottles and is indicated for the prophylaxis of organ rejection in kidney, liver, and heart allogeneic transplants. It is also prescribed for the treatment of chronic rejection in patients previously treated with other immunosuppressive agents.
Distribution Details and Notification Process
The affected lots were distributed exclusively in the US to wholesalers nationwide. Novartis is actively notifying distributors through a recall notification letter and is coordinating the return of the recalled lots from distributors, retailers, and consumers. Health care providers who prescribed this product are also being notified to contact their patients. Consumers with bottles from the recalled lots are advised to reach out to their health care providers.
Consumer Action and Reporting Process
Consumers experiencing adverse reactions or identifying quality issues related to this product are urged to contact their health care provider immediately. Novartis has established a customer interaction center reachable at 888-NOW-NOVA (888-669-6682) from 8:30 AM – 5:00 PM ET, Monday through Friday. Adverse events can also be reported through Novartis’ online reporting platform or via email at usdrugsafety.operations@novartis.com.
Additionally, the recall is being conducted in collaboration with the U.S. Food and Drug Administration (FDA), and consumers are encouraged to report any adverse reactions or quality problems through the FDA’s MedWatch Adverse Event Reporting program. Reports can be submitted online or through regular mail or fax using the information provided on the FDA’s official reporting forms.
Novartis emphasizes its commitment to patient safety and assures that all necessary steps are being taken to address the issue promptly and efficiently. The recall is in alignment with FDA guidelines, and the company remains vigilant in monitoring and ensuring the quality of its pharmaceutical products.




