AI-Powered Smart Bandages on the Horizon
In our daily lives, treating minor wounds with bandages is a routine affair, often taken for granted. However, for more than 8.2 million Americans suffering from chronic wounds, wound care is anything but simple. These chronic wounds, caused by traumatic injuries, post-surgical complications, advanced age, or underlying conditions like diabetes and vascular disease, disrupt the natural healing process, resulting in wounds that persist for months or even years. Alarmingly, untreated chronic wounds can lead to amputations in approximately 30% of cases, and recent studies have revealed that the risk of dying from chronic wound complications within five years rivals that of most cancers.
Despite these dire consequences, the field of wound care has struggled to keep pace with this escalating public health threat. Traditional wound care often involves centuries-old techniques such as poultices and salves, and diagnosing infections remains more art than science. Recognizing the need for innovation, clinicians and researchers, like Dr. Geoffrey Gurtner of the University of Arizona College of Medicine, are transforming the humble bandage through a marriage of cutting-edge materials science, artificial intelligence (AI), and patient data, giving rise to “smart bandages.”
These futuristic bandages, embedded with miniaturized electronics, hold the promise of real-time monitoring of the wound healing process. They can notify patients or healthcare providers when complications arise and can even administer medication or electrical pulses to stimulate healing with a simple press of a smartphone button. Some designs are “closed-loop,” requiring no external prompting, as they continuously monitor the wound and deliver the necessary interventions.
The potential applications of these smart bandages extend beyond chronic wound care. They may prove invaluable in stopping battlefield wounds from hemorrhaging and accelerating healing in blast wounds, thereby preventing long-term disabilities. Moreover, these technologies may also speed up the healing process and minimize scarring in minor cuts and scrapes. Remarkably, these next-generation bandages are not only technologically advanced but could also be produced cost-effectively, benefiting vulnerable populations and potentially saving billions of dollars in chronic wound treatment costs.
Wound healing is a complex biological process, involving clot formation, immune responses, and tissue regeneration. However, conditions like diabetes, poor circulation, and nerve damage can hinder this process, particularly during the inflammatory stage. These setbacks can lead to festering, infected wounds, and significant health risks, making early detection and intervention crucial.
The development of these smart bandages has garnered substantial support, including funding from the Defense Advanced Research Projects Agency (DARPA). Many researchers are racing to create bandages that can monitor wound healing in real-time, using various indicators such as temperature, pH levels, oxygen levels, moisture, glucose, electrical activity, and protein levels. These innovative bandages aim to provide appropriate stimuli to accelerate the healing process based on AI-driven assessments of wound progress.
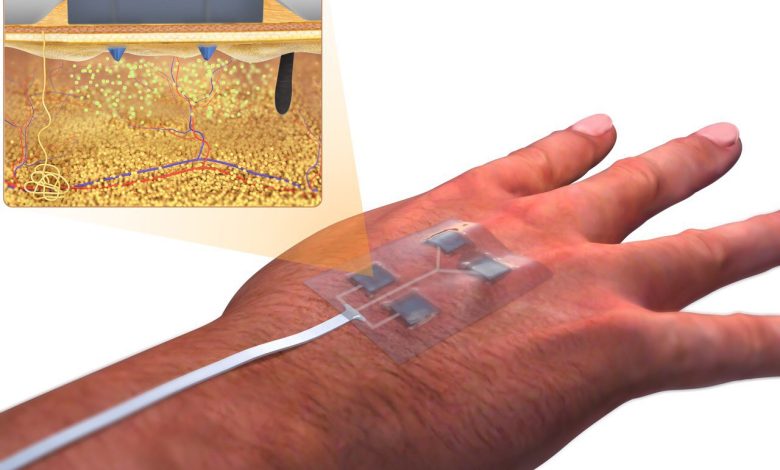
They have made significant strides in the development of “smart bandages” that not only deliver medication to wounds but also monitor the healing process and stimulate tissue repair using a low-level electrical field. These advanced bandages, detailed in a paper published in the journal Science Advances, have the potential to be particularly beneficial for individuals with diabetes, a condition that often hampers wound healing and can lead to more severe complications.
The smart bandages are constructed from a flexible and stretchy polymer that incorporates embedded electronics and medication. These bandages are equipped with sensors capable of monitoring various wound parameters, such as pH levels, temperature, and the presence of molecules like uric acid or lactate, which can indicate inflammation or bacterial infection. Importantly, the device can transmit this real-time data wirelessly to a nearby computer or smartphone for review by either the patient or a healthcare professional. Additionally, the bandage can release antibiotics or other medications directly to the wound site.
One of the notable features of these smart bandages is their ability to create a low-level electrical field that stimulates faster tissue healing, a crucial aspect for chronic wound management. Conditions like diabetic ulcers and burns can persist for extended periods, causing significant challenges for patients. This technology holds great potential for facilitating recovery in such cases.
The research team, led by Wei Gao, an assistant professor of medical engineering at the California Institute of Technology, conducted successful laboratory tests on animal models, demonstrating the bandages’ ability to provide real-time updates on wound conditions. The results were promising, paving the way for further research in collaboration with the University of Southern California’s Keck School of Medicine. Future endeavors will focus on enhancing the bandage technology and conducting tests on human patients, taking into account variations in wound parameters and microenvironments.
Funding for this groundbreaking research came from several organizations, including the National Institutes of Health, the National Science Foundation, the Office of Naval Research, the Heritage Medical Research Institute, and others. The development of these smart bandages represents a significant leap forward in wound care, offering the potential to improve the lives of millions of individuals, particularly those grappling with chronic wounds due to conditions like diabetes.
While some of these developments are still in their early stages and mainly tested on animals, there is optimism that some iteration of smart bandages could be integrated into clinical practice within a few years. These bandages hold the potential to revolutionize wound care, offering an accessible, cost-effective, and technologically advanced solution to a long-ignored medical problem with far-reaching consequences.