Gene Test Identifies Vulnerability to Rare Drug Side Effects in MS and Other Conditions

A significant number of medications used to treat various conditions, including multiple sclerosis, blood cancers, and rheumatoid arthritis, may carry a rare but potentially fatal risk of causing progressive multifocal leukoencephalopathy (PML). New research has revealed that a simple genetic test can identify individuals who have a substantially higher (10-fold) risk of developing PML when using these medications. This information could enable patients and their healthcare providers to explore safer treatment alternatives.
This groundbreaking study represents the most extensive investigation to date into drugs associated with an increased risk of PML and the genetic factors contributing to this condition. Peggy Eis, the chief technology officer at Population Bio, Inc., New York City, and the lead author of the study, emphasized the importance of this research, noting that the elevated risk of drug-induced PML in individuals testing positive for specific genetic variants surpasses some well-known genetic associations used in guiding treatments, such as BRCA1/2 for breast cancer.
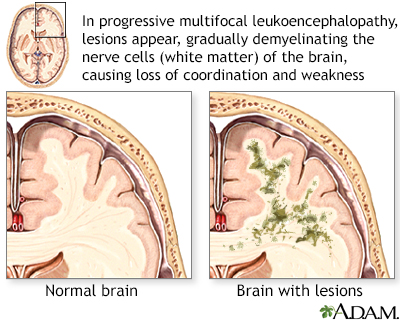
Progressive multifocal leukoencephalopathy (PML) is a neurological disorder characterized by the destruction of cells responsible for producing myelin, a protective substance for nerve cells in the brain. PML is caused by a virus found in up to 85% of the adult population, but it only causes disease when the immune system is significantly weakened. Therefore, it is crucial to identify individuals at higher genetic risk to prevent the onset of PML.
Eis stressed that, since there is currently no cure for PML, prevention through genetic testing is the most effective strategy. The study identified 99 drugs that may pose a risk, particularly for individuals with one of four recently identified genetic variants. These individuals have a tenfold higher risk of developing PML if they use these medications.
In light of these findings, Eis recommends that warning labels on drugs associated with PML should be updated to include a requirement for genetic testing before prescribing these medications. Furthermore, those currently taking these medications should also undergo genetic testing to assess their risk.
The study utilized data from the U.S. Food and Drug Administration Adverse Event Reporting System (FAERS) and identified 81 drugs linked to PML, along with 18 additional drugs in the same class that were not reported to FAERS but could pose similar risks. Many of these medications are immune-suppressing disease-modifying therapies. The researchers investigated whether the drug labels indicated the risk of PML and whether it was classified as a serious adverse event.
Natalizumab and rituximab were found to have the highest number of PML cases, with both drugs carrying a boxed warning. Additionally, the study highlighted that labels on two common blood cancer drugs, daratumumab and venetoclax, did not include any warning regarding the risk of PML.
The genetic variants associated with PML risk are found in four genes (C8B, FCN2, LY9, STXBP2), all of which play essential roles in immune pathways and disorders related to the activation of the JC virus. This virus typically remains dormant in most individuals. However, when it becomes activated in someone with a compromised immune system, it can infect the brain and lead to PML.
For individuals considering these drugs, learning if they have one of these genetic variants can help them explore alternative treatments that do not carry a PML risk, such as interferon-based therapy, glatiramer acetate, or teriflunomide for multiple sclerosis patients. Some patients may still choose to remain on PML-linked therapies due to their effectiveness, even if they test positive for the genetic variants. In such cases, close monitoring, including more frequent brain MRIs, is advisable.
Notably, the test for the four genetic variants associated with PML risk is now available for free in the United States. Patients can take this test at home and send the samples to a lab for analysis.
These study findings were presented at a meeting of the American Neurological Association held in Philadelphia from September 9 – 12, 2023, and should be considered preliminary until they are published in a peer-reviewed journal.