Maxide: Uses, Dosage, Side Effects, Interactions

What is Maxzide (Maxide)?
Maxide (Maxzide) is classified as a thiazide diuretic (water pill), it contains triamterene and hydrochlorothiazide. Maxide helps prevent your body from absorbing too much salt, which can cause fluid retention. Triamterene is a potassium-sparing diuretic that also keeps your potassium levels from getting too low. The combination of hydrochlorothiazide and triamterene is used to treat fluid retention (edema) and high blood pressure (hypertension).
This fixed combination drug is not indicated for the initial therapy of edema or hypertension except in individuals in whom the development of hypokalemia cannot be risked.
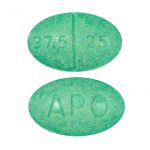
Each Maxzide® (triamterene and hydrochlorothiazide tablets) tablet contains:
Triamterene, USP …………………………………. 75 mg
Hydrochlorothiazide, USP ………………………. 50 mg
Each Maxzide® (triamterene and hydrochlorothiazide tablets) -25 MG tablet contains:
Triamterene, USP ………………………………….. 37.5 mg
Hydrochlorothiazide, USP ……………………….. 25 mg
Maxzide is indicated for the treatment of hypertension or edema in patients who develop hypokalemia on hydrochlorothiazide alone. Maxzide is also indicated for those patients who require a thiazide diuretic and in whom the development of hypokalemia cannot be risked (e.g., patients on concomitant digitalis preparations, or with a history of cardiac arrhythmias, etc.).
Maxzide may be used alone or in combination with other antihypertensive drugs, such as beta-blockers. Since Maxzide may enhance the actions of these drugs, dosage adjustments may be necessary.
Usage In Pregnancy
The routine use of diuretics in an otherwise healthy woman is inappropriate and exposes mother and fetus to unnecessary hazard. Diuretics do not prevent development of toxemia of pregnancy, and there is no satisfactory evidence that they are useful in the treatment of developed toxemia.
Edema during pregnancy may arise from pathological causes or from the physiologic and mechanical consequences of pregnancy. Thiazides are indicated in pregnancy when edema is due to pathologic causes, just as they are in the absence of pregnancy. Dependent edema in pregnancy, resulting from restriction of venous return by the expanded uterus, is properly treated through elevation of the lower extremities and use of support hose; use of diuretics to lower intravascular volume in this case is illogical and unnecessary. There is hypervolemia during normal pregnancy which is harmful to neither the fetus nor the mother (in the absence of cardiovascular disease), but which is associated with edema, including generalized edema, in the majority of pregnant women. If this edema produces discomfort, increased recumbency will often provide relief. In rare instances, this edema may cause extreme discomfort which is not relieved by rest. In these cases, a short course of diuretics may provide relief and may be appropriate.
How is Maxzide used?
The usual dose of Maxzide is one tablet daily, with appropriate monitoring of serum potassium. There is no experience with the use of more than one Maxzide tablet daily or more than two Maxzide-25 MG tablets daily. Clinical experience with the administration of two Maxzide -25 MG tablets daily in divided doses (rather than as a single dose) suggests an increased risk of electrolyte imbalance and renal dysfunction.
Patients receiving 50 mg of hydrochlorothiazide who become hypokalemic may be transferred to Maxzide (triamterene and hydrochlorothiazide) directly. Patients receiving 25 mg hydrochlorothiazide who become hypokalemic may be transferred to Maxzide-25 MG (37.5 mg triamterene/25 mg hydrochlorothiazide) directly.
In patients requiring hydrochlorothiazide therapy and in whom hypokalemia cannot be risked, therapy may be initiated with Maxzide-25 MG. If an optimal blood pressure response is not obtained with Maxzide-25 MG, the dose should be increased to two Maxzide-25 MG tablets daily as a single dose, or one Maxzide tablet daily. If blood pressure still is not controlled, another antihypertensive agent may be added.
What are the side effects of Maxzide?
Common side effects of Maxzide include:
• dizziness,
• lightheadedness,
• headache, or upset stomach as your body adjusts to the medication
Other side effects of Maxzide include:
• nausea,
• diarrhea,
• constipation,
• fatigue,
• headache,
• insomnia, and
• dry mouth
What drugs Interact with maxzide?
Digitalis, lithium toxicity. Adjust antidiabetic, antigout medications. Hyperkalemia is more likely with ACE inhibitors. NSAIDs may cause renal failure. Hypokalemia with corticosteroids, ACTH, amphotericin B. Hypotension with CNS depressants. Potentiates other antihypertensives, and nondepolarizing muscle relaxants. Antagonized by NSAIDs. Hyponatremia with sulfonylureas. Antagonizes folic acid. May block epinephrine. May interfere with parathyroid tests.