Medication Safety
-

How to Insert NuvaRing, Side Effects, Effectiveness, Reviews, FAQ
NuvaRing is a small, bendable contraceptive vaginal ring that has the same working mechanism and contraindications as combined oral contraceptives…
Read More » -

Why is Xanax Being Recalled?
Xanax is a brand of alprazolam an anti-anxiety medication in the benzodiazepine drug family, the same family that includes diazepam…
Read More » -

Why Is Ketoprofen No Longer Available?
Ketoprofen is a nonsteroidal anti-inflammatory drug (NSAID) that is used to treat pain or inflammation caused by arthritis. Prescription ketoprofen…
Read More » -

Why Was Nexium Taken Off The Market?
Nexium is a brand of Esomeprazole magnesium, an FDA-approved medication used as part of combination therapy to treat conditions caused…
Read More » -
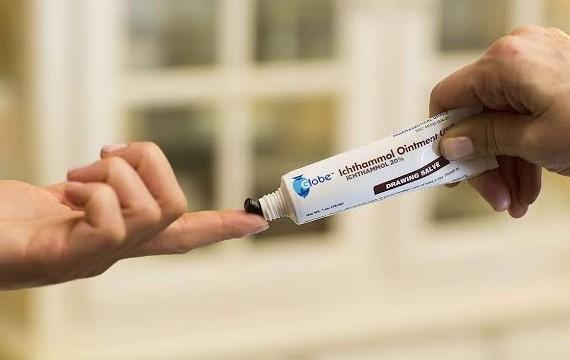
Why is Ichthammol Discontinued?
Ichthammol also referred to as ammonium bituminosulfonate or ammonium bituminosulphonate is an ammonium salt of dark sulfonated shale oil (bituminous…
Read More » -

Are NAC Supplements Banned?
N-acetylcysteine (NAC) also known as Acetylcysteine was synthesized in 1961 and patented by Mead Johnson in 1965. NAC is valued…
Read More » -

When To Take N-acetylcysteine (NAC) Morning Or Night?
What is NAC? N-acetylcysteine (NAC) also known as Acetylcysteine is a medication that is used to treat paracetamol (acetaminophen) overdose,…
Read More » -

How Much NAC Is Safe To Take For COVID-19
What is NAC? N-acetylcysteine (NAC) also known as Acetylcysteine is a medication that is used to treat paracetamol (acetaminophen) overdose,…
Read More » -

Why Was Trilostane Taken Off The Market?
Trilostane, more commonly known by the brand names Modrenal and Vetoryl among others, is a medication that has been used…
Read More » -

Why Was Kenalog Taken Off The Market?
Triamcinolone is a glucocorticoid that is more commonly known by the brand names Kenalog-10 Kenalog-40, Aristocort, Aristocort Forte, Aristospan, Clinacort,…
Read More »
