
Outrun Therapeutics Secures $10M Seed Financing to Advance Protein Stabilization Pipeline
Outrun Therapeutics, a pioneering company in the field of protein stabilization through E3 ligase inhibition, has emerged from stealth mode with a significant milestone:... Read more.

Introducing NexGard SPECTRA®: Boehringer Ingelheim’s Advanced Solution for Dogs Now Available in India
Boehringer Ingelheim, a renowned global leader in animal health, has recently introduced NexGard SPECTRA® (afoxolaner and milbemycin oxime) in India. This significant... Read more.

Central Fill Pharmacy Automation Market Set for Explosive Growth
The Central Fill Pharmacy Automation Market is poised for substantial growth, with an anticipated increase of USD 480.41 million between 2023 and 2027, as per Technavio‘s... Read more.

Kind Pharmaceuticals Resolves Legal Dispute, Advances HIF-PHI Anemia Treatment
Hangzhou Andao Pharmaceutical Ltd. and Kind Pharmaceuticals LLC, alongside their key executives Dr. Dong Liu and Dr. Shaojiang Deng, have reached a resolution in... Read more.
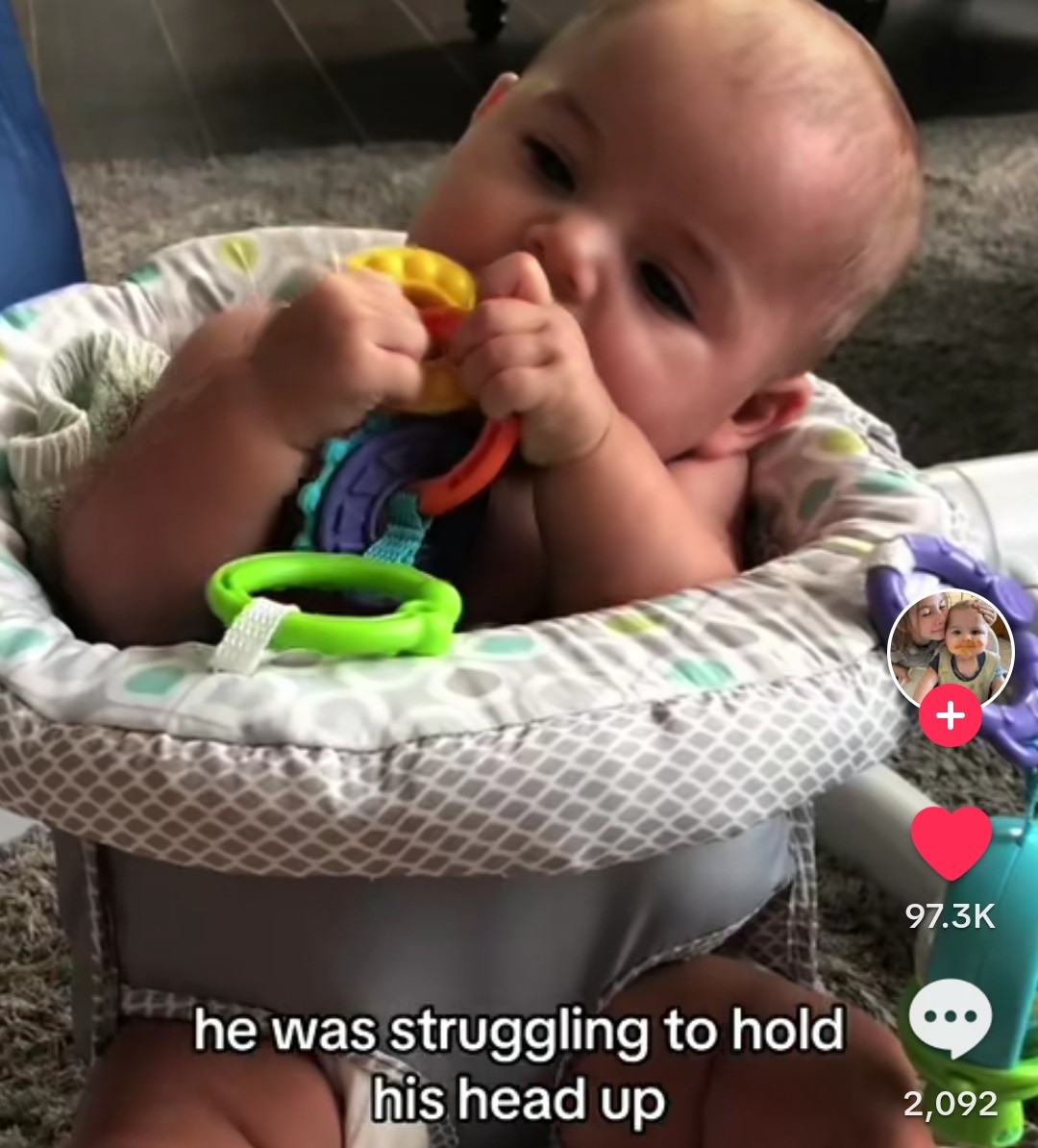
A TikTok Mother’s Journey: Overcoming Mitchell Syndrome with the Wahls Protocol
In a world where challenges seem insurmountable, there are stories that inspire hope and perseverance. One such story is that of a TikTok mother who refused to accept... Read more.

Medical Billing Outsourcing Market Projected to Reach USD 40.1 Billion by 2032 – DataHorizzon Research
The global medical billing outsourcing market is poised for remarkable growth, with projections indicating a surge to USD 40.1 billion by 2032, up from USD 14.1... Read more.
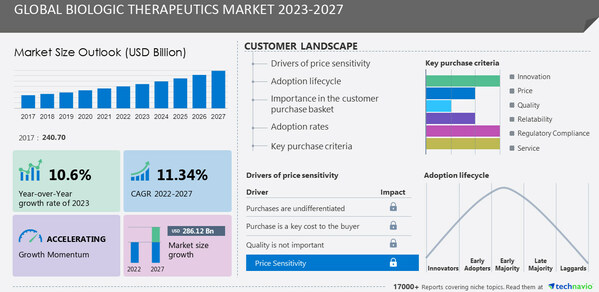
Global Biologic Therapeutics Market Set to Grow by USD 286.12 Billion by 2027, Boosted by Biosimilar Introductions: Technavio
According to the latest analysis by Technavio, the global biologic therapeutics market is anticipated to witness substantial expansion, projecting a growth of USD... Read more.
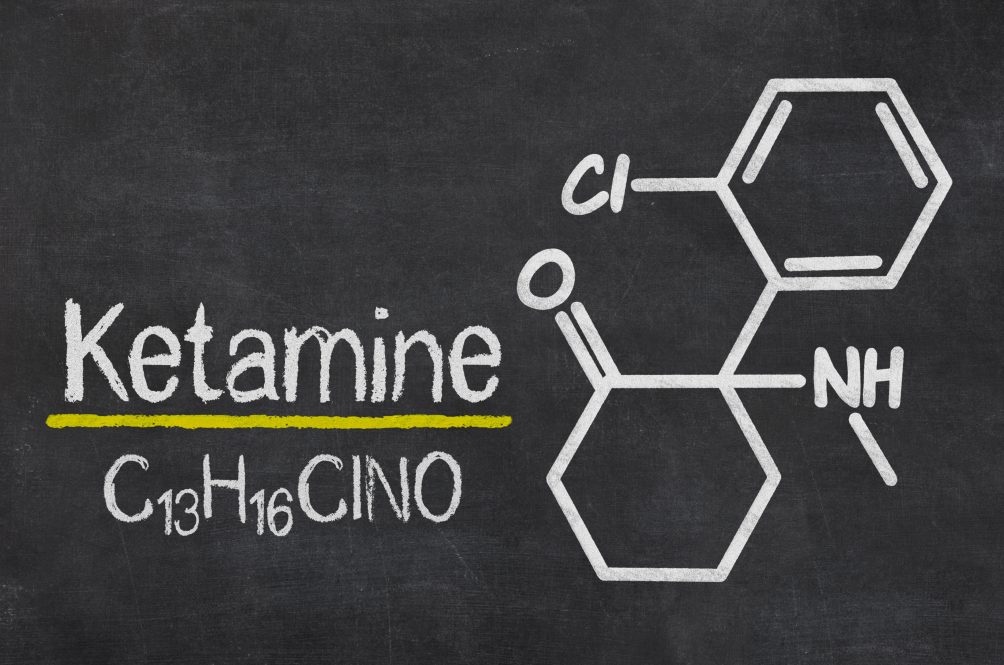
NRx Pharmaceuticals Unveils pH-Neutral IV Ketamine Formulation, HTX-100, Revolutionizing Treatment
NRx Pharmaceuticals, Inc. has made a groundbreaking announcement regarding the development of a new, proprietary formulation of intravenous (IV) ketamine, named... Read more.

Why Amylyx Removed Relyvrio From the U.S. and Canadian Markets
The decision by Amylyx Pharmaceuticals to remove its ALS drug, Relyvrio, from the U.S. and Canadian markets stems primarily from its failure in a crucial late-stage... Read more.
Exploring New Horizons: BCC Research Forecasts $5.2 Billion Global Induced Pluripotent Stem Cells Market by 2028
The global market for induced pluripotent stem cells (iPSCs) is poised for significant growth, according to the latest research study conducted by BCC Research.... Read more.
