Zolgensma: Uses, Side Effects, How it is Administered, Price
What is Zolgensma?
Zolgensma is a prescription gene therapy used to treat children less than 2 years old with spinal muscular atrophy (SMA), a genetic (inherited) neuromuscular disease that causes muscles to become weak and waste away.
People with SMA lose a specific type of nerve cell in the spinal cord (called motor neurons) that control muscle movement. Without these motor neurons, muscles don’t receive nerve signals that make muscles move. The word atrophy is a medical term that means smaller. With SMA, certain muscles become smaller and weaker due to lack of use.
How is Zolgensma Administered?
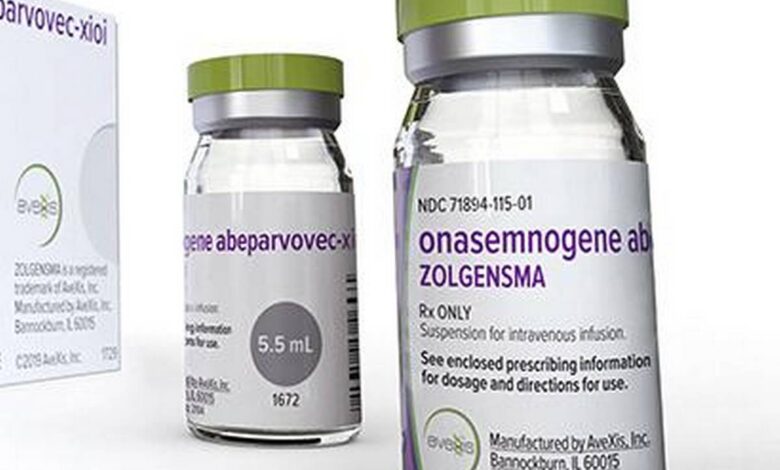
Zolgensma is a suspension for intravenous infusion, supplied as single-use vials. It is provided in a kit containing 2 to 9 vials, as a combination of 2 vial fill volumes (either 5.5 mL or 8.3 mL). All vials have a nominal concentration of 2.0 × 1013 vector genomes (vg) per mL. Each vial of Zolgensma contains an extractable volume of not less than either 5.5 mL or 8.3 mL. Zolgensma is given as a one-time infusion into a vein.
Limitations of Use:
- The safety and effectiveness of repeat administration of Zolgensma have not been evaluated.
- The use of Zolgensma in patients with advanced SMA (e.g.,complete paralysis of limbs, permanent ventilator dependence) has not been evaluated.
What is the most important information I should know about Zolgensma?
Zolgensma can cause acute serious liver injury. Liver enzymes could become elevated and may reflect acute serious liver injury in children who receive Zolgensma. Patients will receive an oral corticosteroid before and after infusion with Zolgensma and will undergo regular blood tests to monitor liver function.
Contact the patient’s doctor immediately if the patient’s skin and/or whites of the eyes appear yellowish, or if the patient misses a dose of the corticosteroid or vomits it up.
What should I watch for before and after infusion with Zolgensma?
Viral respiratory infections before or after Zolgensma infusion can lead to more serious complications. Contact the patient’s doctor immediately if you see signs of a possible viral respiratory infection such as coughing, wheezing, sneezing, runny nose, sore throat, or fever.
Decreased platelet counts could occur following infusion with Zolgensma. Seek immediate medical attention if the patient experiences unexpected bleeding or bruising.
Thrombotic microangiopathy (TMA) has been reported to occur approximately one week after Zolgensma infusion. Caregivers should seek immediate medical attention if the patient experiences any signs or symptoms of TMA, such as unexpected bruising or bleeding, seizures, or decreased urine output.
What do I need to know about vaccinations and Zolgensma?
Talk with the patient’s doctor to decide if adjustments to the vaccination schedule are needed to accommodate treatment with a corticosteroid.
Protection against respiratory syncytial virus (RSV) is recommended.
Do I need to take precautions with the patient’s bodily waste?
Temporarily, small amounts of Zolgensma may be found in the patient’s stool. Use good hand hygiene when coming into direct contact with bodily waste for 1 month after infusion with Zolgensma. Disposable diapers should be sealed in disposable trash bags and thrown out with regular trash.
What are the possible or likely side effects of Zolgensma?
The most common side effects that occurred in patients treated with Zolgensma were elevated liver enzymes and vomiting.
The safety information provided here is not comprehensive. Talk to the patient’s doctor about any side effects that bother the patient or that don’t go away.
You are encouraged to report suspected side effects by contacting the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch, or Novartis Gene Therapies, Inc. at 833-828-3947.
How much does Zolgensma cost?
A one-time gene therapy treatment with Zolgensma costs $2.1 million. Most health insurance systems do not allow for one-time treatments at such a price and a big price tag is also no guarantee that a drug will meet all expectations. Novartis will allow payments over five years, at $425,000 per year, and has said it will give partial rebates if the treatment doesn’t work.
Why is Zolgensma so expensive?
The reason for its exorbitant cost has been linked to its small market size in the drug manufacturing industry and its potential to save lives. “The disorder that Zolgensma treats is rare that is why a highly specialized drug is needed. The expertise required to make Zolgensma and the research around it took a very long time to perfect.
The early development of Zolgensma was financed by the National Institutes of Health and several charities devoted to finding treatments for SMA, including many U.S. charities such as Sophia’s Cure, Cure SMA, Getty Owl Foundation, Fighting SMA, Jadon’s Hope Foundation, the Gwendolyn Strong Foundation, and Miracle for Madison. Many of these charities use donations by patient families and friends to subsidize research and clinical trials into new medicines for SMA.