List of The Most Expensive Drugs In the United States
One major reason given for high prescription drug prices in the United States relative to other countries is the inability of government-granted monopolies in the U.S. health care sector to use their bargaining power to negotiate lower prices and that the US payer ends up subsidizing the world’s R&D spending on drugs.
Studies have shown that Per capita prescription drug spending in the United States exceeds that in all other countries, largely driven by brand-name drug prices that have been increasing in recent years at rates far beyond the consumer price index.
High Cost Medications List
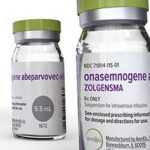
According to a survey report released by telemedicine and pharmacy discount company GoodRx, spinal muscular atrophy treatment Zolgensma is the most expensive drug in the U.S., as the one-time therapy costs $2.1 million.
Below are the 10 most expensive drugs in the U.S., along with their annual cost based on the typical length of therapy:
- Zolgensma ($2,125,000): Zolgensma is a brand-name prescription drug. It’s FDA-approved to treat spinal muscular atrophy (SMA) caused by genetic changes in the SMN1 gene. For this purpose, Zolgensma is given to children less than 2 years old. SMA is a rare condition that damages nerve cells in the brain and spinal cord. This leads to muscle weakness and trouble with activities such as breathing, speaking, swallowing, and walking.
- Zokinvy ($1,032,480): Zokinvy (lonafarnib) is a farnesyltransferase inhibitor indicated in patients 12 months of age and older with a body surface area of 0.39 m2 and above to reduce risk of mortality in Hutchinson-Gilford Progeria Syndrome; and for treatment of processing-deficient progeroid laminopathies with either heterozygous LMNA mutation with progerin-like protein accumulation or homozygous or compound heterozygous ZMPSTE24 mutations.
- Danyelza ($977,664): DANYELZA is a prescription medicine used in combination with a medicine called granulocyte-macrophage colony-stimulating factor (GM-CSF) to treat children 1 year of age and older and adults with high-risk neuroblastoma in the bone or bone marrow that has come back (relapsed) or that did not respond to previous treatment (refractory), and has shown a partial response, minor response, or stable disease to prior therapy.
- Myalept ($889,904): Myalept is a prescription medicine used with a diet recommended by your healthcare provider to treat problems caused by not having enough leptin in the body (leptin deficiency) in people with congenital or acquired generalized lipodystrophy.
- Luxturna ($850,000): Luxturna (voretigene neparvovec-rzyl) is a prescription gene therapy product used for the treatment of patients with inherited retinal disease due to mutations in both copies of the RPE65 gene, which can only be confirmed through genetic testing. You must also have enough remaining cells in your retina (the thin layer of tissue in the back of your eyes) as determined by your healthcare professional.
- Folotyn ($817,865): Folotyn is indicated for the treatment of patients with relapsed or refractory peripheral T-cell lymphoma. The indication for Folotyn is based on overall response rate. Clinical benefit such as improvement in progression free survival or overall survival has not been demonstrated.
- Brineura ($730,340): Brineura (cerliponase alfa) is indicated to slow the loss of ambulation in symptomatic pediatric patients 3 years of age and older with late infantile neuronal ceroid lipofuscinosis type 2 (CLN2), also known as tripeptidyl peptidase 1 (TPP1) deficiency. Brineura is the first enzyme replacement therapy to be directly administered into the fluid of the brain, treating the underlying cause of CLN2 disease by helping to replace the deficient TPP1 enzyme missing in affected children.
- Blincyto ($712,672): Blincyto (blinatumomab) is a prescription medicine used to treat B-cell precursor acute lymphoblastic leukemia (cancer of the blood and bone marrow in which a particular kind of white blood cell is replicating out of control) in patients who still have detectable traces of cancer after chemotherapy.
- Ravicti ($695,970): Ravicti (glycerol phenylbutyrate) binds with other substances in the liver and kidneys to help eliminate nitrogen from the body. Excess nitrogen can cause hyperammonemi, a build-up of ammonia in the blood. Ammonia is very toxic when it circulates in blood and tissues and can cause permanent brain damage, coma, or death.
- Soliris ($678,392): Soliris is a terminal complement inhibitor discovered, developed, and commercialized by Alexion. Soliris works by selectively inhibiting activation of specific proteins in the complement system (C5a and C5b), which play a role in the pathophysiology of multiple rare diseases. It is used in the treatment of patients with paroxysmal nocturnal hemoglobinuria (PNH) to reduce hemolysis, the treatment of patients with atypical hemolytic uremic syndrome (aHUS).