How To Give Deltoid IM Injection
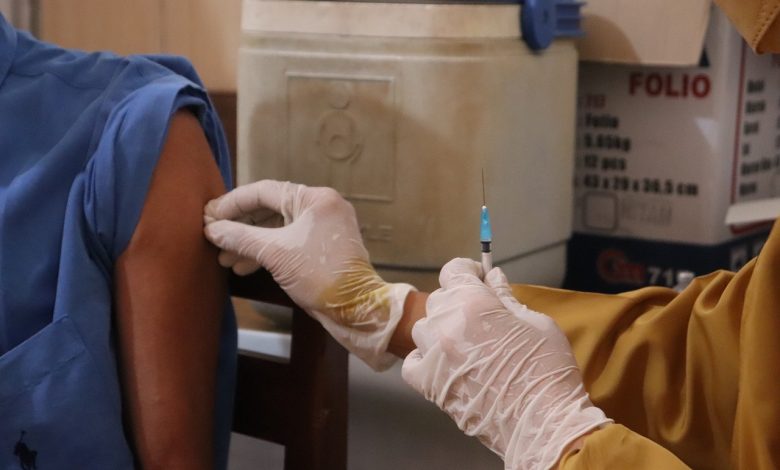
Intramuscular (IM) injections deposit medications into the muscle fascia, which has a rich blood supply, allowing medications to be absorbed faster through muscle fibers than they are through the subcutaneous route. The IM site is used for medications that require a quick absorption rate but also a reasonably prolonged action.
Due to their rich blood supply, IM injection sites can absorb larger volumes of solution, which means a range of medications, such as sedatives, anti-emetics, hormonal therapies, analgesics, and immunizations, can be administered intramuscularly in the community and acute care setting. In addition, muscle tissue is less sensitive than subcutaneous tissue to irritating solutions and concentrated and viscous medications.
The technique of IM injections has changed over the past years due to evidence-based research and changes in equipment available for the procedure. An IM site is chosen based on the age and condition of the patient and the volume and type of medication injected. When choosing a needle size, the weight of the patient, age, amount of adipose tissue, medication viscosity, and injection site all influence the needle selection.
The deltoid is a large rounded triangular shape on the outside of the upper arm, on top of the humerus, the clavicle, and the scapula. The brachial artery and the radial nerve sit behind the deltoid and come down from the muscle.
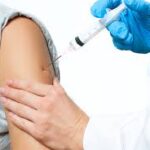
According to drugs.com, a deltoid IM injection is given into a specific area of the deltoid muscle. Many vaccinations are given by this route. There are several different ways to find the deltoid muscle injection site, which is the central and thickest portion of the deltoid muscle, for example:
• Find the acromion process, which is the bony bit that sticks out from your shoulder, just above the deltoid muscle. Approximately 2–3 fingerbreadths (approximately 2 inches) below the acromion process, and sitting just above the level of the armpit, in the central part of the upper arm is the deltoid injection site.
• Find the intersection of an imaginary line drawn between the anteroposterior axillary line and a perpendicular line from the mid-lateral of the acromial process (imagine where a line drawn from the middle of the acromion process would bisect a line drawn across the top level of the armpit).
To aid identification of the muscle area, you can ask the person to raise their arm first to define the muscle. Once defined, have the patient relax their arm and proceed.
To avoid causing an injury, do not inject too high (near the acromion process) or too low (which may miss the muscle).
How do you give a deltoid IM injection?
1. Locate the deltoid injection site, as described above.
2. Use a needle long enough to reach the deep muscle. For vaccinations in adults, this is usually a 22–25-gauge needle which is 1 inch (25mm) long for those weighing less than 70kg (154lbs), 1 to 1.5 inches (25-38mm) long for those 70-90kg (154-198lbs), and 1.5 inches (38mm) long in those more than 90kg (198lbs).
3. Use your thumb and index finger to stretch the skin around the injection site.
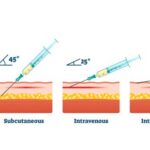
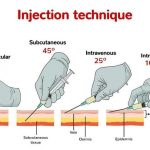
4. Insert the needle with a deep thrust at a 90-degree angle to the skin (straight up and down). Aspiration is not necessary or recommended. Push down on the plunger of the syringe slowly, then withdraw the needle once all the contents have been administered.
5. Apply a plaster or wound pad to the area if there is any bleeding.
6. If you are giving two deltoid IM injections into the same arm, separate them by a minimum of one inch.
Do you pinch the skin when giving a deltoid IM injection?
No, you should not pinch the skin if you are giving a deltoid IM injection because this may mean you inject the syringe contents into the subcutaneous tissue rather than the muscle.
What angle do you give an IM deltoid injection?
You should insert the needle at a 90-degree angle to the skin (straight up and down) to ensure it gets into the deltoid muscle.
Deltoid IM injection must be done carefully to avoid complications. Complications with IM injections include muscle atrophy, injury to bone, cellulitis, sterile abscesses, pain, and nerve damage. With IMs, there is an increased risk of injecting the medication directly into the patient’s bloodstream. In addition, any factors that impair blood flow to the local tissue will affect the rate and extent of drug absorption. Because of the adverse and documented effects of pain associated with IM injections, always use this route of administration as the last alternative; consider other methods first.