US WorldMeds Receives FDA Approval for Iwilfin, a Revolutionary Maintenance Therapy for High-Risk Neuroblastoma
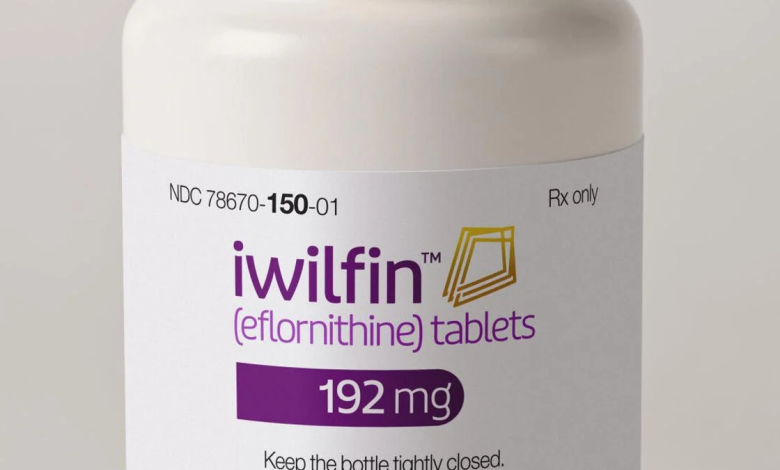
In a significant stride towards improving outcomes for high-risk neuroblastoma patients, US WorldMeds (USWM, LLC) has secured approval from the U.S. Food and Drug Administration (FDA) for Iwilfin™ (eflornithine) 192 mg tablets. This oral maintenance therapy, designed to reduce the risk of relapse in both adult and pediatric populations, is a transformative addition to the treatment landscape for high-risk neuroblastoma.
Key Highlights:
• Indication and Prevalence: Iwilfin is indicated for patients with high-risk neuroblastoma who have shown at least a partial response to prior multiagent, multimodality therapy, including anti-GD2 immunotherapy. Neuroblastoma, diagnosed in 700-800 cases annually in the U.S., with 90% occurring before age 5, witnesses over 50% of cases classified as high-risk.
• Efficacy and Study Basis: The FDA approval is rooted in the results of a multi-site, single-arm, externally controlled study, demonstrating notable improvements in event-free survival (EFS) and overall survival (OS) in high-risk neuroblastoma patients treated with Iwilfin. This includes a 52% reduction in the risk of relapse and a 68% reduction in the risk of death.
• Administration and Tolerance: Administered orally, Iwilfin is taken twice daily for two years. It is generally well-tolerated, with manageable side effects such as hearing loss, otitis media, pyrexia, pneumonia, and diarrhea.
• Availability: Anticipated to be available to patients in the United States in the coming weeks, Iwilfin holds promise as a much-needed treatment option for children battling high-risk neuroblastoma.
Industry Impact and Collaborations
US WorldMeds collaborated with the Beat Childhood Cancer Research Consortium at Penn State University for preclinical and clinical research, a partnership pivotal in navigating Iwilfin through the FDA registration process. The approval of Iwilfin is seen as a significant advancement in the battle against high-risk neuroblastoma, offering newfound hope for pediatric patients facing this devastating disease.
How stakeholders reacted
• Breck Jones, Chief Executive Officer of US WorldMeds: “We are thrilled to announce the FDA approval of Iwilfin, which provides a new and much-needed treatment option for children with high-risk neuroblastoma.”
• Kristen Gullo, Vice President of Development and Regulatory Affairs at US WorldMeds: “This FDA approval represents a beacon of hope for the high-risk neuroblastoma community and a significant step forward in the fight against this devastating disease.”
• Giselle Saulnier Sholler, MD, Chair and Founder, Beat Childhood Cancer Research Consortium: “After diligent efforts for the past decade, I am both humbled and overjoyed to see this product that holds the potential to evolve the standard of care for high-risk neuroblastoma come to fruition.”
Important Safety Information
Patients considering Iwilfin are advised to consult their healthcare providers regarding medical conditions, pregnancy, breastfeeding, and potential side effects such as low blood cell counts, liver problems, and hearing loss.
About US WorldMeds
US WorldMeds, a privately held specialty pharmaceutical company, focuses on developing, licensing, and marketing healthcare products designed to address challenging conditions and unmet medical needs.