What is The Normal Co-codamol 30/500mg Dose?
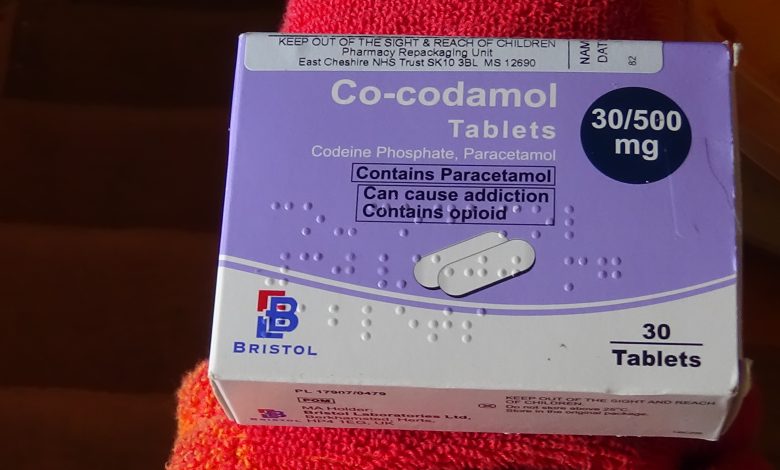
Co-codamol 30/500mg is a medication commonly prescribed for pain relief, containing two active ingredients: codeine phosphate (30mg) and paracetamol (500mg). This combination provides an effective way to manage moderate to severe pain.
Co-codamol is commonly prescribed to alleviate pain resulting from various conditions, such as injuries, surgical procedures, dental work, or chronic conditions causing persistent pain. The codeine component in co-codamol acts as an opioid analgesic, while paracetamol enhances the pain-relieving effects and helps reduce fever.
How Long Does Co-codamol Take To Work?
The onset of action of co-codamol, like many pain relievers, can vary from person to person. Co-codamol is a combination medication containing codeine phosphate and paracetamol (acetaminophen), and both components have different mechanisms of action.
• Paracetamol (Acetaminophen): This component typically begins to work relatively quickly, often within 30 minutes to an hour after ingestion. It works by reducing the production of prostaglandins in the brain, which are chemicals that cause pain and fever.
• Codeine Phosphate: Codeine is an opioid analgesic that changes the way the brain and nervous system respond to pain. It generally has a slower onset of action compared to paracetamol and may take around 30 minutes to 1 hour to start providing pain relief.
The combined effect of these two components in co-codamol can result in a more comprehensive and quicker pain relief compared to each ingredient alone. However, individual responses can vary based on factors such as metabolism, the severity of pain, and the presence of other medical conditions.
Dosage Guidelines
The recommended dosage for co-codamol 30/500mg may vary based on individual factors such as the severity of pain, the patient’s medical history, and their response to the medication. In general, a typical dosage is one or two tablets taken every 4 to 6 hours as needed for pain relief. It’s essential to follow the specific instructions provided by healthcare professionals or those outlined on the medication label.
Here is the dosage breakdown according to the patient information leaflet:
- Adults: 2 tablets every 4 to 6 hours up to a maximum of 8 tablets in 24 hours. Elderly people may be prescribed a lower dose.
- Use in children aged 16 to 18 years: 1-2 tablets every 6 hours when necessary up to a maximum of 8 tablets in 24 hours.
- Use in children aged 12 to 15 years: 1 tablet every 6 hours when necessary to a maximum of 4 tablets in 24 hours.
This medicine should not be taken for more than 3 days. If the pain does not improve after 3 days, talk to your doctor for advice.
Co-codamol should not be given to children under 12 years of age due to the risk of severe breathing problems.
Precautions and Considerations
1. Avoid Alcohol: Patients taking co-codamol should avoid alcohol consumption. The combination of codeine and alcohol can lead to increased drowsiness and respiratory depression.
2. Driving and Machinery: Co-codamol may cause drowsiness, affecting the ability to drive or operate machinery. It is crucial to assess individual reactions before engaging in activities that require mental alertness.
3. Kidney and Liver Function: Individuals with impaired kidney or liver function should exercise caution and consult their healthcare provider before taking co-codamol. Adjustments to the dosage may be necessary in such cases.
4. Pregnancy and Breastfeeding: Pregnant or breastfeeding individuals should consult their healthcare provider before using co-codamol, as it may have implications for the unborn child or nursing infant.
Side Effects
While co-codamol is generally well-tolerated when used as directed, it may cause side effects in some individuals. Common side effects may include dizziness, drowsiness, constipation, and nausea. If these effects persist or worsen, it’s crucial to seek medical attention promptly.
Conclusion
Co-codamol 30/500mg is a valuable medication for managing pain when used appropriately and under the guidance of healthcare professionals. Patients must adhere to prescribed dosages, be aware of potential side effects, and communicate any concerns or adverse reactions to their healthcare provider. As with any medication, the benefits and risks should be carefully considered, and personalized medical advice sought when needed.