G 3719 Pill: Uses, Dosage, Side Effects, Warnings
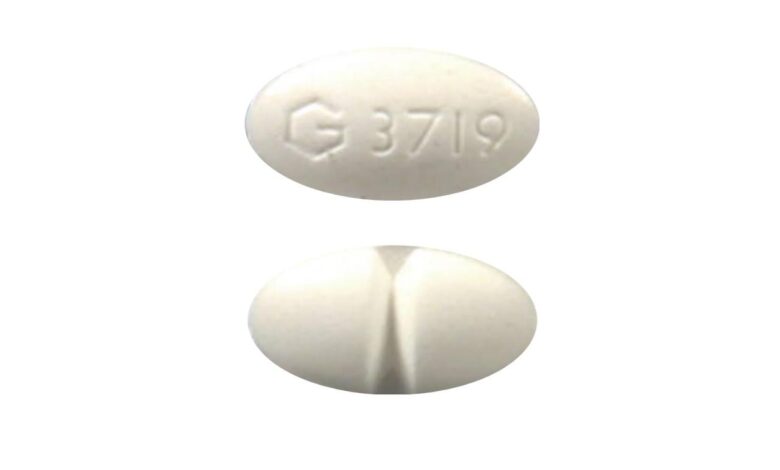
What does the G 3719 pill contain?
The white oval pill with the imprint G 3719 has been identified as Alprazolam 0.25 mg. It is supplied by Greenstone Limited. Alprazolam is classified as a benzodiazepine. (A drug’s classification refers to a group of medications that work in the same way.) G 3719 pill is used to treat anxiety disorders and panic disorder (sudden, unexpected attacks of extreme fear and worry about these attacks). G 3719 pill works by decreasing abnormal excitement in the brain.
Your doctor may have suggested this medication for conditions other than those listed in these drug information articles. If you have not discussed this with your doctor or are not sure why you are taking this medication, speak to your doctor. Do not stop taking this medication without consulting your doctor.
Do not give this medication to anyone else, even if they have the same symptoms as you do. It can be harmful for people to take this medication if their doctor has not prescribed it.
How should I use G 3719 pill?
The recommended dose of G 3719 pill for adults varies depending on its use. The dose is usually started low and increased if necessary, as prescribed by your doctor. It is important that the dose be individualized to your specific needs to avoid excessive sedation or motor impairment.
Many things can affect the dose of medication that a person needs, such as body weight, other medical conditions, and other medications. If your doctor has recommended a dose different from the ones listed here, do not change the way that you are taking the medication without consulting your doctor.
G 3719 pill may be habit-forming when taken for long periods of time. If you have been taking this medication regularly for a long period of time (i.e., more than one month), do not stop taking the medication without speaking with your doctor. A gradual reduction in dose is recommended when stopping this medication to avoid withdrawal symptoms.
It is important to take this medication exactly as prescribed by your doctor. If you miss a dose, take it as soon as possible and continue with your regular schedule. If it is almost time for your next dose, skip the missed dose and continue with your regular dosing schedule. Do not take a double dose to make up for a missed one. If you are not sure what to do after missing a dose, contact your doctor or pharmacist for advice.
Store at room temperature in a dry place and keep it out of reach of children.
Do not dispose of medications in wastewater (e.g. down the sink or in the toilet) or in household garbage. Ask your pharmacist how to dispose of medications that are no longer needed or have expired.
How long does G 3719 pill take to kick in?
G 3719 pill is taken by mouth and absorbed quickly by the bloodstream. Some people may first begin experiencing the effects of G 3719 pill within 5 to 10 minutes of taking the pill. Almost everyone will feel the effects of the drug within an hour. One of the reasons why G 3719 pill is so effective for treating panic is that peak impact from the dose comes quickly. Most people will experience it between one and two hours after taking their dose.
Who should NOT take G 3719 pill?
Do not take this medication if you:
• are allergic to Alprazolam or any ingredients of the medication
• are allergic to any other medications belonging to the benzodiazepine class
• have acute narrow-angle glaucoma (G 3719 pill may be used by people with open-angle glaucoma who are receiving appropriate treatment)
• have myasthenia gravis
• have severe breathing problems
• have sleep apnea
• have severely reduced liver function
• are taking the medications ketoconazole or itraconazole
What are the side effects of G 3719 pill?
Many medications can cause side effects. A side effect is an unwanted response to a medication when it is taken in normal doses. Side effects can be mild or severe, temporary or permanent.
The side effects listed below are not experienced by everyone who takes this medication. If you are concerned about side effects, discuss the risks and benefits of this medication with your doctor.
The following side effects have been reported by at least 1% of people taking this medication. Many of these side effects can be managed, and some may go away on their own over time.
Contact your doctor if you experience these side effects and they are severe or bothersome. Your pharmacist may be able to advise you on managing side effects.
• blurred vision or other changes in vision
• changes in sexual desire or ability
• clumsiness or unsteadiness
• constipation
• dizziness or lightheadedness
• drowsiness
• false sense of well-being
• increased appetite
• problems with urination
• unusual tiredness
• weight changes
Although most of these side effects listed below don’t happen very often, they could lead to serious problems if you do not seek medical attention.
Check with your doctor as soon as possible if any of the following side effects occur:
• abnormal thinking (e.g., disorientation, delusions, or loss of sense of reality)
• agitation
• anxiety
• behaviour changes (e.g., aggressive behaviour, bizarre behaviour, decreased inhibition, or outbursts of anger)
• confusion
• increased risk of falls (particularly for seniors)
• loss of recent memory
• signs of depression (e.g., poor concentration, changes in weight, changes in sleep, decreased interest in activities, thoughts of suicide)
• signs of liver problems (e.g., nausea, vomiting, diarrhea, loss of appetite, weight loss, yellowing of the skin or whites of the eyes, dark urine, pale stools)
• slurred speech
• symptoms of mania (e.g., decreased need for sleep, elevated or irritable mood, racing thoughts)
• trouble sleeping
Stop taking the medication and seek immediate medical attention if any of the following occur:
• signs of a serious allergic reaction (e.g., abdominal cramps, difficulty breathing, nausea and vomiting, or swelling of the face and throat)
• signs of a severe skin reaction such as blistering, peeling, a rash covering a large area of the body, a rash that spreads quickly, or a rash combined with fever or discomfort
• signs of increased pressure in the eyes (e.g., sudden severe eye pain, changes in side vision, decreased or cloudy vision, seeing halos around lights, swollen eyes)
Some people may experience side effects other than those listed. Check with your doctor if you notice any symptom that worries you while you are taking this medication.
Are there any other precautions or warnings for this medication?
Before you begin using a medication, be sure to inform your doctor of any medical conditions or allergies you may have, any medications you are taking, whether you are pregnant or breast-feeding, and any other significant facts about your health. These factors may affect how you should use this medication.
Alcohol and other medications that cause drowsiness: People taking this medication should not combine it with alcohol and avoid combining it with other medications, such as narcotic pain relievers, that cause drowsiness. Doing so can cause additive drowsiness and reduced breathing as well as other side effects, which can be dangerous and possibly fatal.
Dependence and withdrawal: Physical dependence (a need to take regular doses to prevent physical symptoms) has been associated with benzodiazepines such as G 3719 pill. Severe withdrawal symptoms may be experienced if the dose is significantly reduced or suddenly discontinued. These symptoms include seizures, irritability, nervousness, sleep problems, agitation, tremors, diarrhea, abdominal cramps, vomiting, memory impairment, headache, muscle pain, extreme anxiety, tension, restlessness, and confusion. Reducing the dose gradually, under medical supervision, can help prevent or decrease these withdrawal symptoms.
Because the treatment of panic disorder often requires the use of average daily doses of G 3719 pill above 3 mg, the risk of dependence among people with panic disorder may be higher than that among those treated for less severe anxiety.
Drowsiness/reduced alertness: G 3719 pill may affect the mental or physical abilities needed to drive or operate machinery. Do not engage in activities requiring mental alertness, judgment, and physical coordination until you have established how G 3719 pill affects you. Alcohol can increase the drowsiness effects of G 3719 pill and should be avoided.
Kidney function: Kidney disease or reduced kidney function may cause this medication to build up in the body, causing side effects. If you have kidney problems, discuss with your doctor how this medication may affect your medical condition, how your medical condition may affect the dosing and effectiveness of this medication, and whether any special monitoring is needed.
Liver function: Liver disease or reduced liver function may cause this medication to build up in the body, causing side effects. If you have liver problems, discuss with your doctor how this medication may affect your medical condition, how your medical condition may affect the dosing and effectiveness of this medication, and whether any special monitoring is needed.
Mood changes: Anxiety disorders are often associated with mood changes, such as depression, mania, or ideas of suicide or self-harm. If you have depression or a history of depression, discuss with your doctor how this medication may affect your medical condition, how your medical condition may affect the dosing and effectiveness of this medication, and whether any special monitoring is needed.
If you experience symptoms of depression such as poor concentration, changes in weight, changes in sleep, decreased interest in activities, or notice them in a family member who is taking this medication, contact your doctor as soon as possible.
Pregnancy: Other benzodiazepine medications similar to G 3719 pill are known to increase the risk of birth defects when taken by a woman who is pregnant. The safety of G 3719 pill during pregnancy has not been established. It is not recommended for use by pregnant women.
Breast-feeding: G 3719 pill may pass into breast milk. If you are a breast-feeding mother and are taking G 3719 pill, it may affect your baby. Talk to your doctor about whether you should continue breast-feeding.
Children and adolescents: The safety and efficacy of G 3719 pill for children and adolescents under 18 years of age have not been established.
Seniors: Seniors may be at increased risk of experiencing side effects such as sedation, reduced coordination, and dizziness. Use extra caution when rising from a sitting or lying position to reduce the risk of severe dizziness and falls.
What other drugs could interact with this medication?
There may be an interaction between G 3719 pill and any of the following:
• alcohol
• antihistamines (e.g., cetirizine, doxylamine, diphenhydramine, hydroxyzine, loratadine)
• antipsychotics (e.g., chlorpromazine, clozapine, haloperidol, olanzapine, quetiapine, risperidone)
• antiseizure medications (e.g., carbamazepine, clobazam, gabapentin, levetiracetam, phenobarbital, phenytoin, primidone, topiramate, valproic acid, zonisamide)
• apalutamide
• aprepitant
• azelastine
• “azole” antifungals (e.g., ketoconazole, itraconazole, fluconazole)
• barbiturates (e.g., butalbital, phenobarbital)
• benzodiazepines (e.g., diazepam, lorazepam)
• bosentan
• bicalutamide
• birth control pills
• brimonidine
• buprenorphine
• buspirone
• calcium channel blockers (e.g., amlodipine, diltiazem, nifedipine, verapamil)
• cannabis
• chloral hydrate
• cobicistat
• conivaptan
• deferasirox
• dexamethasone
• dofetilide
• dronedarone
• entacapone
• enzalutamide
• flibanserin
• general anesthetics (medications used to put people to sleep before surgery)
• grapefruit juice
• HIV non-nucleoside reverse transcriptase inhibitors (NNRTIs; e.g., delavirdine, efavirenz, etravirine, nevirapine)
• HIV protease inhibitors (e.g., atazanavir, indinavir, ritonavir, saquinavir)
• kava kava
• lomitapide
• lumacaftor
• macrolide antibiotics (e.g., erythromycin, clarithromycin)
• magnesium sulfate
• melatonin
• methadone
• mifepristone
• mirtazapine
• mitotane
• muscle relaxants (e.g., baclofen, cyclobenzaprine, methocarbamol, orphenadrine, tizanidine)
• modafinil
• nabilone
• narcotic pain relievers (e.g., codeine, fentanyl, morphine, oxycodone)
• olopatadine
• pramipexole
• rifabutin
• rifampin
• ropinirole
• rotigotine
• St. John’s wort
• sarilumab
• scopolamine
• selective serotonin reuptake inhibitors (SSRIs; e.g., citalopram, fluoxetine, paroxetine, sertraline)
• siltuximab
• sodium oxybate
• stiripentol
• tapentadol
• tetracycline
• thalidomide
• theophylline
• tocilizumab
• tramadol
• trazodone
• tricyclic antidepressants (e.g., amitriptyline, desipramine, imipramine)
• tyrosine kinase inhibitors (e.g., ceritinib, crizotinib, dabrafenib, dasatinib, imatinib)
• valerian
• yohimbine
• zolpidem
• zopiclone
If you are taking any of these medications, speak with your doctor or pharmacist. Depending on your specific circumstances, your doctor may want you to:
• stop taking one of the medications,
• change one of the medications to another,
• change how you are taking one or both of the medications, or
• leave everything as is.
An interaction between two medications does not always mean that you must stop taking one of them. Speak to your doctor about how any drug interactions are being managed or should be managed.
Medications other than those listed above may interact with this medication. Tell your doctor or prescriber about all prescription, over-the-counter (non-prescription), and herbal medications you are taking. Also tell them about any supplements you take. Since caffeine, alcohol, the nicotine from cigarettes, or street drugs can affect the action of many medications, you should let your prescriber know if you use them.





