Signs of Blistering Defects In Tablet Coatings

Tablet coating is a process in which a thin layer of coating material is applied to the surface of tablets. The coating serves various purposes, including protection, improved appearance, ease of swallowing, taste masking, and controlled release.
The tablet coating process typically involves the following steps:
1. Preparation: The tablet cores, which contain the active pharmaceutical ingredient (API) and other excipients, are manufactured through processes such as granulation or compression. The tablet cores should be clean, dry, and free from dust or contaminants before coating.
2. Coating Formulation: The coating formulation is prepared by mixing the coating material with other ingredients such as plasticizers, colorants, and solvents. The specific formulation depends on the desired characteristics of the coated tablets, including properties like taste, appearance, release profile, and stability.
3. Coating Equipment Setup: The coating equipment is set up and adjusted according to the specific coating process parameters. This includes ensuring proper calibration of the equipment, adjusting airflow, spray rate, and temperature settings.
4. Coating Application: The tablet cores are loaded into a coating pan or drum within the coating equipment. The coating material is then applied to the tablet surface through a process known as spraying. The coating material is atomized into fine droplets and sprayed onto the tablet cores while they are continuously agitated and tumbled in the coating pan.
5. Drying: After the coating material is applied, the tablets go through a drying process to remove the solvent and allow the coating to solidify. The drying may involve controlled airflow, heating, or the use of specific drying chambers. It is essential to ensure proper drying conditions to prevent issues such as sticking, uneven coating, or inadequate film formation.
6. Inspection and Quality Control: Once the coating process is complete, the coated tablets undergo inspection and quality control checks. This includes visual inspection to assess the appearance, color, and uniformity of the coating, as well as conducting tests for weight variation, disintegration, dissolution, and other quality parameters.
Different coating techniques and equipment can be used depending on the scale of production and specific requirements. Some common coating methods include pan coating, fluidized bed coating, and coating using specialized equipment like perforated coating pans or tablet coaters.
It’s important to note that the coating process should be conducted in compliance with Good Manufacturing Practices (GMP) to ensure the quality, safety, and efficacy of the coated tablets.
The selection of coating materials, process parameters, and equipment depends on factors such as the specific medication, desired coating characteristics, regulatory requirements, and manufacturing capabilities. Pharmaceutical manufacturers continually innovate and refine coating techniques to improve the performance and patient acceptability of coated tablets.
Blistering Defects
Blistering is a defect that can occur in coated tablets, where the coating material forms bubbles or blisters on the tablet surface. This defect can impact the appearance, integrity, and functionality of the tablets. Here are some factors that can contribute to blistering defects in tablets:
1. Moisture: Excessive moisture, either in the tablet core or in the coating material, can cause blistering. Moisture trapped within the tablet core can migrate to the surface during the coating process, leading to blister formation. Similarly, if the coating solution contains excess moisture, it can create bubbles when sprayed onto the tablet surface.
2. Inadequate Drying: Insufficient drying time or inadequate drying conditions can result in blistering. If the tablets are not thoroughly dried before packaging, residual moisture can become trapped within the coating, causing blisters.
3. Incompatibility: Incompatibility between the tablet core and the coating material can lead to blistering. Certain interactions between the tablet formulation and the coating solution can cause gas generation, resulting in the formation of bubbles or blisters.
4. Coating Process Parameters: Improper control of coating process parameters such as spray rate, atomization pressure, temperature, and airflow can contribute to blistering defects. Inadequate atomization or excessive spray rate can lead to uneven coating application and the formation of blisters.
5. Coating Material Selection: The choice of coating material can also influence blistering. Some coating materials may be more prone to blister formation due to their inherent characteristics or incompatibility with the tablet formulation.
6. Equipment-related Issues: Malfunctioning or inadequate calibration of the coating equipment can contribute to blistering defects. Improper adjustment of airflow, spray gun position or drying conditions can result in uneven coating and the formation of blisters.
To address blistering defects in coated tablets, manufacturers can take several measures:
• Optimize coating process parameters, such as spray rate, drying time, and temperature, to ensure proper coating application and drying.
• Conduct thorough validation and testing of the coating process to identify and rectify potential issues.
• Use appropriate coating materials that are compatible with the tablet formulation and provide good film-forming properties.
• Implement quality control measures to inspect the coated tablets for blistering defects and ensure adherence to specifications.
• Conduct stability studies to assess the long-term performance and integrity of the coated tablets.
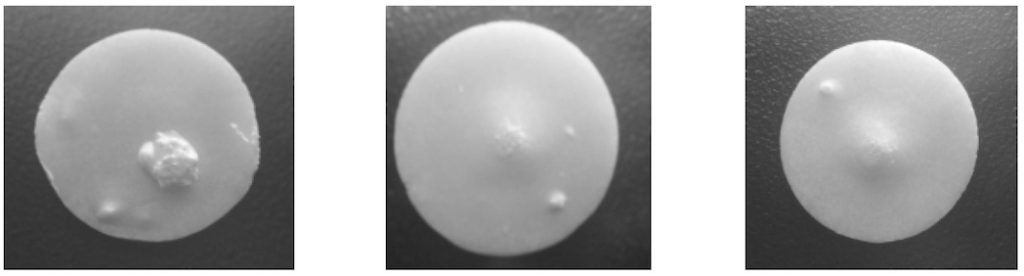
Signs of Blistering Defect Tablets In Tablet Coating
Blistering is a defect that can occur in the coating of tablets, where the coating material forms bubbles or blisters on the tablet surface. These blisters can vary in size and may affect the appearance, integrity, and functionality of the coated tablets. Here are some signs that indicate the presence of blistering defects in coating tablets:
1. Bubbles or Swollen Areas: Blistering defects manifest as bubbles or swollen areas on the tablet surface. These bubbles can be small or large, localized or widespread, and may appear as raised regions on the tablet coating.
2. Irregular Texture: Blistering can cause an irregular or bumpy texture on the tablet surface. The affected areas may feel rough or have an uneven appearance compared to the surrounding coated regions.
3. Cracks or Openings: In some cases, the blisters may rupture, resulting in cracks or openings in the tablet coating. These cracks can expose the underlying tablet core, potentially compromising the stability and functionality of the medication.
4. Changes in Color or Opacity: Blistering defects can also lead to changes in color or opacity of the affected areas. The blistered regions may appear discolored, faded, or have a different hue compared to the rest of the tablet surface.
5. Reduced Tablet Integrity: Blistering can weaken the structural integrity of the tablet coating, making it more prone to damage or disintegration during handling, transportation, or packaging. The tablets may become more fragile or susceptible to crumbling.
It is important to note that blistering defects can be caused by various factors, including formulation issues, improper coating technique, excessive moisture, inadequate drying, or interactions between tablet ingredients and the coating material.
What to do when your tablets have coating problems?
As a patient, if you notice any issues with the coating of your tablets, here’s what you can do:
1. Document the Problem: Take note of the specific issue you are experiencing with the tablet coating. This can include issues like rough texture, peeling, unusual taste, or any other observable problems.
2. Contact Your Healthcare Provider: Reach out to your healthcare provider, such as your doctor or pharmacist, and inform them about the problem you’ve noticed with the tablet coating. Describe the issue in detail and provide any relevant information, such as the medication name, batch number, and expiration date.
3. Follow Guidance: Follow the guidance provided by your healthcare provider. They may ask you to bring the affected tablets for inspection or provide alternative medication options if necessary.
4. Do Not Alter Medication: It is important not to alter the tablets yourself by attempting to remove or modify the coating. The coating may serve a specific purpose, such as protecting the active ingredient or facilitating controlled release, and altering it can affect the medication’s effectiveness or safety.
5. Report the Issue: Consider reporting the tablet coating problem to the appropriate regulatory authority in your country. In the United States, you can report adverse events or quality concerns to the Food and Drug Administration (FDA) through their MedWatch program. Similarly, other countries have their own reporting systems to address such concerns.
6. Store Medications Properly: Ensure that you are storing your medications according to the instructions provided, including temperature and humidity conditions. Improper storage can potentially impact the stability of the coating and the overall quality of the tablets.
Remember, your healthcare provider is your primary resource for addressing medication concerns. They can guide you on the appropriate steps to take and ensure your safety and well-being.





