Onfi: Uses, Mechanism of Action, Dosage, Side Effects, Interactions
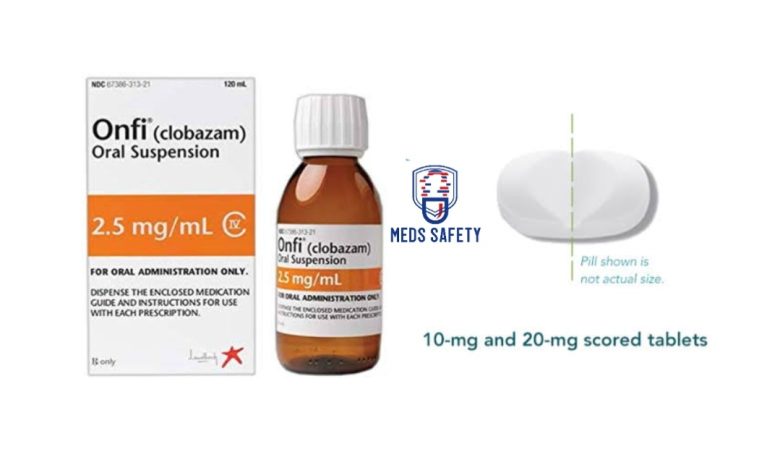
Onfi, known generically as clobazam, is a medication that falls under the class of benzodiazepines. It is primarily prescribed as an adjunctive treatment for individuals with Lennox-Gastaut syndrome (LGS), a severe form of epilepsy.
Onfi is specifically approved for the treatment of seizures associated with Lennox-Gastaut syndrome in patients two years of age and older. Lennox-Gastaut syndrome is a severe and rare form of epilepsy that typically appears in childhood. It is characterized by multiple types of seizures, cognitive impairment, and abnormal electroencephalogram (EEG) patterns. Onfi was specifically developed to address the seizures associated with this syndrome.
The efficacy of Onfi in the treatment of seizures associated with LGS was demonstrated in clinical trials. These studies showed that Onfi, when used as an adjunctive treatment, significantly reduced the frequency of seizures compared to placebo. As a result, it received FDA approval as an additional treatment option for patients with LGS. Onfi is used as an adjunctive therapy alongside other antiepileptic medications to help control seizures and improve quality of life.
This article aims to provide an overview of Onfi, its mechanism of action, approved uses, potential side effects, and other important considerations.
Mechanism of Action
The exact mechanism of action of Onfi is not fully understood, but it is believed to exert its antiepileptic effects through its activity on the gamma-aminobutyric acid (GABA) system in the brain.
GABA is an inhibitory neurotransmitter that plays a crucial role in regulating neuronal excitability. It functions by binding to specific receptor sites, known as GABA-A receptors, which are ion channels that allow chloride ions to enter neurons, thereby hyperpolarizing the cell membrane and reducing neuronal excitability.
Onfi enhances the effects of GABA by binding to specific sites on the GABA-A receptors, which are distinct from the GABA binding sites. This binding increases the frequency of chloride channel opening, leading to enhanced inhibitory effects in the brain. This hyperpolarization of neurons helps to reduce the excessive electrical activity that underlies seizures.
In addition to its activity on GABA-A receptors, Onfi is also metabolized in the liver by cytochrome P450 enzymes, specifically CYP3A4 and CYP2C19. These enzymes convert clobazam to its active metabolite, N-desmethylclobazam (N-CLB), which contributes to the antiepileptic effects of the medication. N-CLB has a longer half-life than Onfi itself, allowing for sustained therapeutic activity.
The precise contributions of clobazam and its metabolite to the overall therapeutic effects of Onfi are not fully elucidated. However, both compounds are believed to play a role in the modulation of neuronal excitability and the reduction of seizure activity.
Dosage and Administration
Onfi is available in tablet or oral suspension form for convenient administration. The dosage of Onfi is determined by a healthcare professional based on individual needs, taking into account factors such as age, weight, and other medications. It is typically started at a low dose and gradually increased to find the optimal therapeutic effect. The following guidelines are typically followed:
1. For individuals weighing less than 30 kg (66 pounds), the initial dose of Onfi is usually 5 mg per day. The dosage can be increased as directed by a healthcare professional, up to a maximum of 20 mg per day.
2. For individuals weighing more than 30 kg (66 pounds), the initial dose of Onfi is typically 10 mg per day. The dosage can be increased as directed by a healthcare professional, up to a maximum of 40 mg per day.
• Onfi is generally taken twice a day.
• The dosage is usually increased gradually, either once a week or at a slower pace, to reach the target dose.
• It takes approximately 5 to 9 days for the body to reach a stable state with the new dose.
When taking clobazam tablets:
• Swallow the tablets whole with at least half a glass of water.
• If needed, the tablets can be split in half along the score or crushed and mixed with soft foods like applesauce.
• Clobazam can be taken with or without food.
Important considerations:
• It is crucial not to abruptly stop taking Onfi without medical guidance, as this can lead to seizures that may not cease, as well as other withdrawal symptoms such as hallucinations, tremors, anxiety, and stomach or muscle cramps.
Using the Onfi oral suspension:
• Shake the bottle well before each dose if you are taking the Onfi oral suspension.
• Measure the dose using the bottle adapter and dosing syringes provided with the suspension.
Potential Side Effects
Like other benzodiazepines, Onfi may cause certain side effects, although their severity and occurrence can vary from person to person. Common side effects may include drowsiness, sedation, dizziness, fatigue, and difficulties with coordination. It is important to discuss any concerns or side effects with a healthcare provider.
Precautions and Considerations
Before starting Onfi, it is crucial to inform your healthcare provider about any existing medical conditions, such as liver or kidney problems, as dosage adjustments may be necessary. Onfi can cause drowsiness and impair cognitive function, so activities requiring mental alertness, such as driving or operating machinery, should be approached with caution. Additionally, sudden discontinuation of Onfi should be avoided, as it may lead to withdrawal symptoms.
Onfi Interactions with Other Medication
Like any medication, Onfi can potentially interact with other drugs, including prescription medications, over-the-counter drugs, and herbal supplements. It’s important to inform your healthcare provider about all the medications you are taking, including Onfi, to avoid any potential interactions. Here are some notable interactions:
1. Central Nervous System Depressants: Onfi can enhance the central nervous system depressant effects of other medications that act on the brain, such as sedatives, tranquilizers, sleep aids, and opioids. Taking Onfi with these drugs can increase the risk of excessive sedation, drowsiness, respiratory depression, and other side effects. It’s important to use caution and follow your healthcare provider’s instructions.
2. Medications that affect liver enzymes: Onfi is metabolized by liver enzymes, specifically cytochrome P450 (CYP450) enzymes. Certain medications can either increase or decrease the activity of these enzymes, potentially affecting the levels of Onfi in your body. Examples include rifampin, phenytoin, carbamazepine, and phenobarbital. Your healthcare provider may need to adjust your Onfi dosage if you start or stop any of these medications.
3. Birth Control Pills: Onfi can reduce the effectiveness of hormonal contraceptives, such as birth control pills, patches, or rings. This interaction may increase the risk of pregnancy. It is recommended to use an additional non-hormonal method of contraception, such as condoms, while taking Onfi.
4. Anti-seizure Medications: Onfi may interact with other antiepileptic drugs you may be taking. Your healthcare provider will carefully consider the potential interactions and adjust the dosages of these medications accordingly to ensure optimal seizure control.
5. Grapefruit and Grapefruit Juice: Consuming grapefruit or grapefruit juice while taking Onfi may increase the levels of the drug in your bloodstream, leading to an increased risk of side effects. It’s best to avoid grapefruit products while on Onfi.
These are just a few examples of potential interactions. Always consult your healthcare provider or pharmacist for a comprehensive list of medications, supplements, or foods that may interact with Onfi based on your specific situation. They will be able to provide you with personalized advice and guidance.
Monitoring and Follow-up
Regular medical check-ups and monitoring are essential while taking Onfi. These appointments allow healthcare professionals to assess the medication’s effectiveness, adjust dosage if necessary, and address any concerns or side effects that may arise.
How to Store
To properly store Onfi, follow these guidelines:
- Tablets: Keep them at room temperature in a dry location, away from moisture and excessive heat. Store them in a place where children cannot access them.
- Oral Suspension Bottle: After opening the bottle, ensure that you tightly close the cap to prevent air and moisture from entering. Store the bottle in an upright position to maintain the integrity of the medication.
- Shelf Life of Oral Suspension: The Onfi oral suspension should be used within 90 days after first opening the bottle. It is important to keep track of the date you first opened the bottle. Discard any remaining suspension after this 90-day period.
By following these storage instructions, you can help maintain the stability and effectiveness of Onfi throughout its intended shelf life.