ISMP Issues Safety Alert on Pfizer’s Paxlovid

The Institute for Safe Medication Practices has issued a safety alert warning pharmacists of potential error risks with Paxlovid, Pfizer’s COVID-19 antiviral pill regimen. The alert states patients with severe renal impairment should not receive the drug, and patients with moderate renal impairment should receive a dose reduction.
Paxlovid is administered as three tablets (two tablets of nirmatrelvir and one tablet of ritonavir) taken twice daily for no more than five consecutive days. For patients with moderate renal impairment, pharmacists should remove one of the nirmatrelvir tablets for both the morning and evening doses from each of the five blister cards before dispensing, the ISMP alert said, adding that the empty blisters should be covered with manufacturer-supplied stickers.
ISMP said additional potential safety issues include patients accidentally failing to take both tablets together and drug interactions.
The full copy of the ISMP alert is presented below:
On December 22, 2021, the US Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for PAXLOVID, consisting of oral tablets of nirmatrelvir that are co-packaged with oral tablets of ritonavir (an FDA-approved antiretroviral agent).
Indications
Emergency use of Paxlovid is indicated for the treatment of mild-to-moderate coronavirus disease 2019 (COVID-19) in adults and pediatric patients (12 years of age and older weighing at least 40 kg) with positive results of direct severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral testing, and who are at high risk for progression to severe COVID-19, including hospitalization or death. Paxlovid is not authorized for the treatment of hospitalized patients, or for use as pre- or post-exposure prophylaxis for prevention of COVID-19. Paxlovid is not indicated for use longer than 5 consecutive days.
Dosing
Nirmatrelvir is available in 150 mg tablets, while ritonavir is a 100 mg tablet. For patients with normal renal function or mild renal impairment, the recommended dose is 300 mg of nirmatrelvir (two tablets) and 100 mg of ritonavir (one tablet), taken together, twice daily, in the morning and evening, for 5 days.
Dose Reduction
A dose reduction is necessary for patients with moderate renal impairment, defined as having an estimated glomerular filtration rate (eGFR) below 60 mL/minute, but more than or equal to 30 mL/minute. If patients have moderate renal impairment, they must only receive 150 mg of nirmatrelvir (one tablet) along with 100 mg of ritonavir, taken together, twice daily, in the morning and evening. Patients with severe renal impairment, with an eGFR below 30 mL/minute, should not receive the drug, as the appropriate dosage for patients with severe renal impairment has not been determined.
Important Note for Dispensing Pharmacists
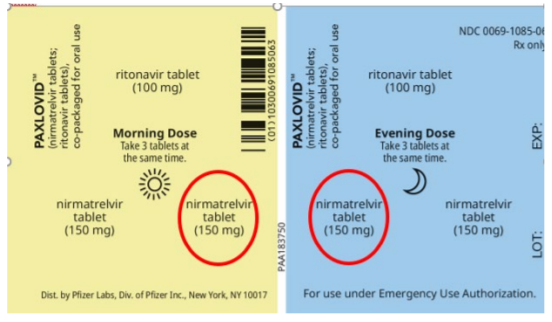
Paxlovid is only available in a carton holding five blister cards, each containing the daily morning and evening doses (two nirmatrelvir tablets and one ritonavir tablet for each dose) for patients with normal renal function or mild renal impairment. For patients with moderate renal impairment, the EUA directs pharmacists to remove one of the nirmatrelvir tablets for both the morning and evening doses from each blister card before dispensing Paxlovid to facilitate proper dosing (Figure 1). After removing one nirmatrelvir tablet from the morning dose and one from the evening dose on each blister card, the empty blisters on all five cards should be covered with manufacturer-supplied stickers (Figure 2). Pharmacies needing additional stickers should contact: C19therapies@amerisourcebergen.com. It is essential for pharmacists to take these steps, as outlined in the EUA dispensing information for patients with moderate renal impairment.

Safety Concerns and Recommendations
Challenges with prescribing the dose. The prescriber may not be aware that the dose should be reduced for moderate renal impairment or that the drug should not be prescribed for patients with severe renal impairment. Thus, electronic prescribing systems should alert the prescriber to renal dosing requirements. Also, choosing the correct dose for patients with moderate renal impairment may require prescribers to manually enter the reduced dose in a text field. It is critical for prescriptions to specify the numeric dose of each active ingredient in Paxlovid as follows:
- 150 mg of nirmatrelvir with 100 mg of ritonavir for patients with moderate renal impairment
- 300 mg of nirmatrelvir with 100 mg of ritonavir for patients with normal renal function or mild renal impairment
Failure to remove tablets and cover empty blisters. One significant safety concern is that pharmacy staff may fail to remove one of the nirmatrelvir tablets from each dose of the blister card for all five days of therapy, and/or may miss applying the stickers to make patients aware that the packaging has been altered to remove unneeded tablets. Both of these steps are a requirement under the EUA, and pharmacies handling Paxlovid must ensure their pharmacists and pharmacy technicians address this issue and have a process in place to meet this requirement.
Failure to take the tablets together. Patients will be self-administering Paxlovid at home. Thus, it is extremely important for pharmacists to counsel patients to take both the nirmatrelvir and ritonavir tablets together in the morning and evening. For patients with moderate renal impairment, pharmacists should also explain that the packaging has been altered to provide the proper dose.
Drug Interactions
Paxlovid (nirmatrelvir co-packaged with ritonavir) is an inhibitor of CYP3A, the most abundant clinically significant group of cytochrome P450 isoenzymes, which may increase plasma concentrations of drugs that are primarily metabolized by CYP3A. At the same time, nirmatrelvir and ritonavir are CYP3A substrates; therefore, drugs that induce CYP3A may decrease nirmatrelvir and ritonavir plasma concentrations and reduce the therapeutic effect of Paxlovid. For complete information and a list of serious drug interactions and expected effects, please refer to the Paxlovid Fact Sheet for Healthcare Providers.
Mandated Reporting
Providers must report all serious adverse events or medication errors potentially related to Paxlovid to the FDA MedWatch reporting program, which is mandatory for medications available under an EUA. Please also fax a copy of the MedWatch form to Pfizer (8666358337). ISMP also asks providers to report errors to the ISMP National Medication Errors Reporting Program (ISMP MERP).
The Institute for Safe Medication Practices (ISMP) is an independent, nonprofit agency dedicated to medication safety. ISMP accepts no advertising in its publications or other work product. ISMP is an affiliate of ECRI, an independent, nonprofit organization improving the safety, quality, and cost‐effectiveness of care across all healthcare settings worldwide.