Why Is Sinutab Off The Market?
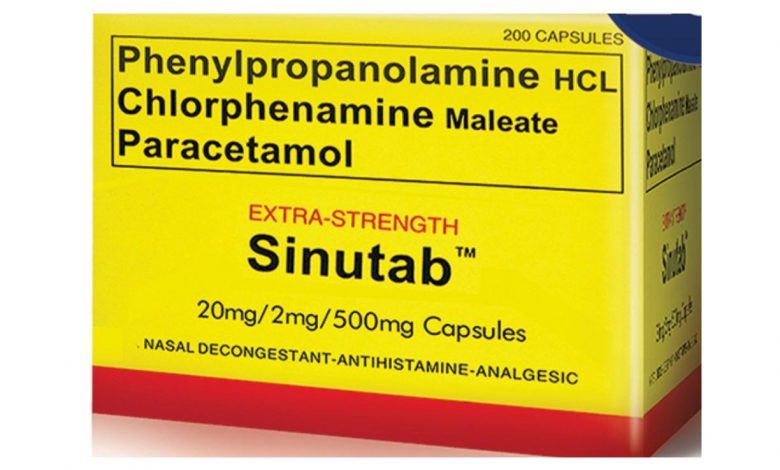
Sinutab is a combination medication used to temporarily treat symptoms caused by the common cold, flu, allergies, or other breathing illnesses (such as sinusitis, bronchitis). Sinutab was originally marketed by Warner–Lambert. It is manufactured and distributed by Johnson & Johnson after its acquisition of Pfizer’s consumer healthcare division in late December 2006.
Currently, Sinutab is packaged as white, round, biconvex, uncoated tablets, with each tablet containing 30 mg of pseudoephedrine hydrochloride (PSE), 500 mg of paracetamol (acetaminophen), and 2 mg of chlorpheniramine maleate (CPM) as the active ingredients.
Why Was Sinutab Off The Market?
Sinutab originally contained Phenylpropanolamine (20mg), Chlorpheniramine maleate (2mg), paracetamol (500mg) but in the year 2000, Sinutab became a subject of controversy after a five-year study by scientists at Yale University reported that Phenylpropanolamine, an ingredient in the product, was associated with an increased risk of hemorrhagic stroke (bleeding into the brain or into tissue surrounding the brain) in women and that men may also be at risk.
After a review, the Administration (FDA) recommended that consumers should avoid such products until the ingredient has been replaced and to check labels carefully. “We don’t want to be alarmist, but it is important for consumers to know there are real risks associated with taking this ingredient. The adverse effects are rare, but they can be fatal while the conditions treated by the ingredient are not,” said Charles Ganley, director of the FDA’s non-prescription drug division. He said the FDA’s action was not a recall, but a request for drug companies to voluntarily take products with PPA off the market. The agency would propose a formal new rule limiting the use of PPA.
In issuing the sweeping public health advisory, officials at the drug agency went one step beyond the recommendation of an outside panel of experts, which recently called for PPA to be removed from over-the-counter drugs. Instead, the agency asked the manufacturers of all PPA products, 100 companies in all, to stop marketing them voluntarily while regulators draft new rules that would effectively ban the ingredient.
The Yale University study, published in The New England Journal of Medicine, found that women ages 18 to 49 who took phenylpropanolamine as an appetite suppressant were as much as 15 times more likely than other women to suffer hemorrhagic stroke, a type of stroke characterized by bleeding in the brain. First-time users of the ingredient were three times as likely to suffer a stroke.
A panel of scientific experts reviewed the study and, in a series of votes, said phenylpropanolamine could no longer be ”generally recognized as safe and effective,” the agency’s standard for over-the-counter products. It did not consider whether the ingredient should be allowed in prescription drugs.
Safety concerns about phenylpropanolamine first emerged in the early 1980s, when the food and drug agency began receiving reports that some people taking the chemical had suffered hemorrhagic stroke. In 1990, Senator Ron Wyden, Democrat of Oregon, then a congressman, held hearings about the ingredient, and the following year the F.D.A. held a public meeting to examine the risks.