How to Spot Fake Pills With Nuclear Quadrupole Resonance

When you purchase medicine at the drugstore, you assume that it’s what you think it is and that the active ingredient in the drug is present in the specified concentration. Unfortunately, your assumption might be all wrong. Counterfeit and substandard medicines have become widespread, particularly in low- and middle-income countries with weak regulatory systems. Indeed, according to the World Health Organization (WHO), one out of 10 medicines sold in developing countries should be considered “substandard.” Your drug could even be an outright fake.
“But I live in the United States,” you may say. “The medicines at my pharmacy are regulated by the U.S. Food and Drug Administration, so it must be the genuine article.” Unfortunately, even the United States and other higher-income countries aren’t immune to this scourge. Since 2012, smugglers have been caught selling fake drugs to more than 3,000 doctors, clinics, and hospitals across the United States.
In one notorious case, two lots of the cancer drug Avastin were discovered to contain none of that medicine’s active ingredient. After a recall and lengthy investigation, the FDA concluded that the fake Avastin had traveled through a network of overseas suppliers, passing through Canada before reaching the United States.
And even if your drugs aren’t counterfeit, what assurance do you have that the pharmaceutical ingredients have not degraded? Many drugs are sensitive to heat, and neither you nor your pharmacist has any way of telling whether the pills you’ve just picked up experienced problematically high temperatures—say, in the back of the truck that delivered them.
The growing use of online pharmacies is only making these problems worse. In the United States, tens of millions of people (about 8 percent of the adult population) buy medicines outside the formal drug supply chain, typically from foreign online pharmacies or other unlicensed sources. A quarter of the people in the United Kingdom say they’re likely to use an online pharmacy in the near future.
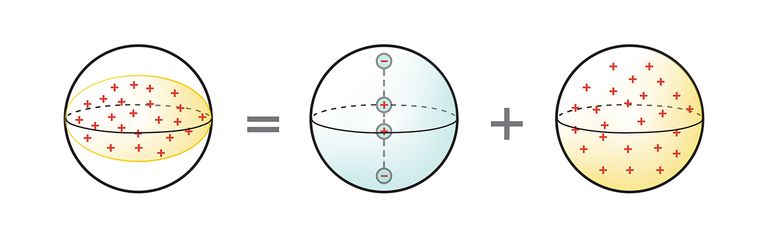
Four Poles: Nuclear quadrupole resonance requires an atomic nucleus with a nonspherical distribution of positive electric charge (left), which creates an electric quadrupole moment. The word quadrupole refers to the four electric poles (center) that produce an equivalent nonspherical charge distribution when added to a set of spherically distributed charges (right).ILLUSTRATION: ERIK VRIELINK
The issue of fake and substandard medicines has attracted the attention of law enforcement authorities around the world. In 2008, Interpol created Operation Pangea, a special division targeting the online sale of illegal pharmaceuticals; last year it seized 10 million units of fake and illicit medicines and shut down more than 3,600 websites. And hundreds of millions of doses are seized every year at international borders. The global market in counterfeit drugs is estimated to be worth somewhere between US $75 billion and $200 billion annually. People are wasting a lot of money buying this useless stuff.
But forget the economic losses: The human toll is what really matters, and it is enormous. Ineffective antimicrobial drugs in particular compromise the treatment of many deadly diseases, especially in poor countries. They also promote development of antimicrobial resistance. WHO estimates that 72,000 to 169,000 children die each year from pneumonia due to substandard or counterfeit antibiotics. And in sub-Saharan Africa, an estimated 64,000 to 158,000 people die every year from malaria that they tried to treat with substandard or fake antimalarial medications.
It’s no exaggeration to compare illicit medicines with the nuclear and biological weapons of mass destruction we all fear. These WMDs, though, are largely aimed at people in poor countries who are already facing a multitude of social and economic ills.
Clearly, we need better ways to detect medicines that are fake, substandard, or degraded from negligent handling. Efforts to improve the situation include recent provisions of the European Union’s Falsified Medicines Directive, adopted last year. As of last February, all packaged medicines transported into and across the EU must now have a “unique medicines identifier”—a separate reference number for every packet of medicine, not just every brand or batch—and also a tamper-evident seal. In the United States, the Drug Supply Chain Security Act, signed into law in 2013, requires all packages of medicine to be electronically traceable by 2023.
Sadly, these “track and trace” approaches suffer from a fundamental shortcoming: They authenticate the package, not its contents. A package of medicine is assumed to be genuine simply because it has a valid security mark. Unscrupulous manufacturers can readily circumvent such measures by putting the wrong stuff in the right package. And these approaches offer no help in detecting degradation.
Our research groups at Case Western Reserve University, in Cleveland, and the University of Florida, in Gainesville, along with some colleagues at King’s College London, have been looking at a technology that promises to be a whole lot more effective—offering a way to authenticate the actual contents of a packet of medicine. You might imagine that any system used to verify the chemical constitution of a dose of medicine would necessarily destroy it. In fact, though, using a physical phenomenon called nuclear quadrupole resonance (NQR), you can test your pill and eat it, too.
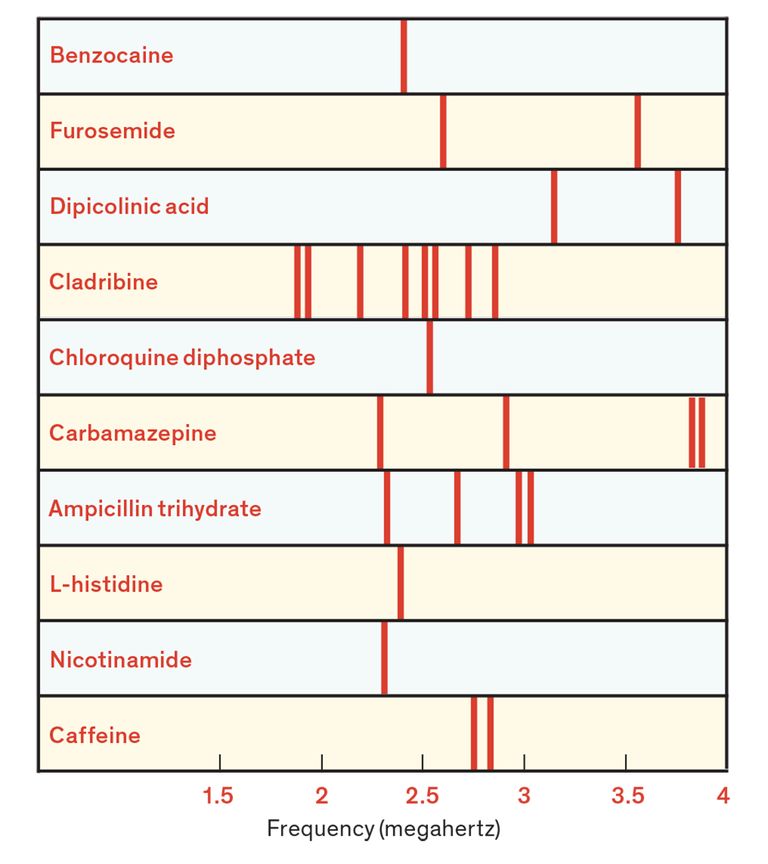
NQR provides a relatively easy and nondestructive way to perform a chemical analysis on many kinds of packaged medicines and dietary supplements. The electronic tester we’ve been developing measures the NQR response of the sample at different frequencies. The resulting NQR spectrum is generated by energy transitions within the atomic nuclei of the chemical, providing a unique fingerprint for that compound. While the quantum mechanics of the phenomenon is complicated, here’s what’s basically going on at the atomic level.
Atomic nuclei contain positively charged particles (protons), usually along with electrically neutral particles (neutrons). If the distribution of positive charges is not perfectly symmetrical, the nucleus will have what is called a charge moment, which characterizes how the charge is distributed. In the nuclei of some atoms, the positive charge, rather than being spherically distributed, stretches toward two poles of the nucleus, creating what is known as an electric quadrupole moment. (It’s not possible for a nucleus to have an electric dipole moment.) Or the positive charge could accumulate around the “equator” of the nucleus, which would also give rise to a quadrupole moment (but of the opposite sign).
Nuclei with quadrupole moments can occupy only certain distinct energy levels. Those levels are determined by the interaction between a nucleus’s quadrupole moment and the charge distribution of the electrons that surround the nucleus. That charge distribution is in turn determined by the chemical environment the nucleus finds itself in. So by measuring the energy levels in the nucleus—or more accurately, by measuring the differences between energy levels as the nucleus shifts from one level to another—you can infer what the chemical environment is.
About half of the elements in the periodic table have some isotopes with nuclear quadrupole moments. Many of those isotopes are rare. Fortunately, the most common isotopes of nitrogen (nitrogen-14) and chlorine (chlorine-35 and chlorine-37) have nuclear quadrupole moments, and many medicines contain one or both of these kinds of atoms.
When subjected to radio waves of between 0.1 and 5 megahertz, nitrogen nuclei will shift energy levels, absorbing and later reemitting radio energy at distinct frequencies that depend on the chemical and, to some extent, the physical environment of those nuclei. Chlorine nuclei do the same thing, but at frequencies from 20 to 40 MHz.
NQR spectra in these frequency bands can thus serve as unique chemical fingerprints for nitrogen- and chlorine-containing compounds. By measuring such spectra, you can readily identify many kinds of drugs. Indeed, in some cases NQR spectra can even be used to distinguish between identical drugs prepared in slightly different ways—say, tablets containing different inactive ingredients or produced using machines that apply different compacting pressures. Our NQR-based drug-testing apparatus has, for example, been able to reliably classify tablets of acetaminophen, an over-the-counter pain killer, made by different manufacturers.
Nuclear quadrupole resonance is useful for testing specimens that are solids or powders, but not liquids. While that’s an obvious limitation, NQR has a lot of other things going for it. In particular, it’s insensitive to the presence of coatings or packaging materials. So it can be used to examine pills while they’re still in the bottle or blister pack. Indeed, it could be used to test an entire shipping carton of such bottles or packs, or a drum of powdered material.
What’s more, the equipment could be built at low cost and would be amenable to miniaturization. And because NQR instrumentation relies on radio waves of relatively low frequency and power, it is inherently safe and could be used without special training.

Inner Works: The authors’ prototype instrument for measuring nuclear quadrupole resonance contains a simple coil (right, with pills inside) and two analog signal-processing boards (left and center). Digital signal processing is done by circuitry located in a second box.PHOTO: SWARUP BHUNIA AND SOUMYAJIT MANDAL
You may have noticed the similarity between the terms nuclear quadrupole resonance and the more familiar nuclear magnetic resonance, which is the physical phenomenon on which magnetic resonance imaging (MRI) is based. Indeed, there are many similarities between the two phenomena: Both are sensitive to the presence of certain kinds of atomic nuclei, both detect transitions between nuclear energy levels by measuring radio signals in the megahertz range, and both can be used to produce images.
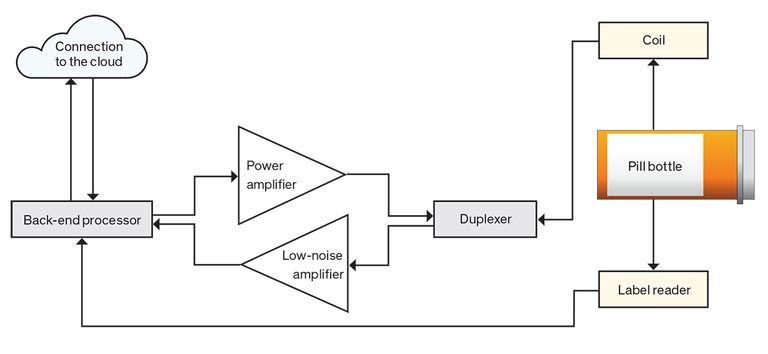
A key difference, though, is that NQR doesn’t require that the sample be placed in a strong magnetic field. So there’s no need for an expensive superconducting magnet of the type found in MRI scanners. All you really need is a coil of wire and some suitable electronics for generating the appropriate radio-frequency excitations and measuring the sample’s response.
The excitation consists of one or more RF pulses generated by a power amplifier producing at most a few watts. These pulses are applied to the sample by a transmitter coil that generates an oscillating magnetic field. The field gives rise to oscillations within some of the atomic nuclei in the sample; the oscillations can be measured by a receiving coil attached to a suitable RF amplifier. As a result, the equipment is pretty simple. And you can simplify it further by including a switch that allows you to use the same physical coil for both transmission and reception.
The electronics do have to be sophisticated enough to amplify and process the acquired NQR signal and extract parameters of interest: signal amplitude, frequency, phase, and decay rate. In the instrument we have built, an embedded computer compares these parameters against reference data to classify the sample. Our prototype drug-authentication device is portable, performs measurements automatically, and doesn’t require any special skills to operate. It could thus be used anywhere in the drug supply chain. We estimate such a device could be manufactured at a cost of about $100, which would, presumably, translate to a price for the end user of less than $1,000.
In our view, NQR holds much more promise than any other analytical technique you could imagine. Liquid chromatography and mass spectrometry, for example, while effective at identifying chemical compounds, involve expensive apparatus and necessitate destroying the sample. Optical methods, such as near-infrared or Raman spectroscopy, don’t have those drawbacks, but they’re able to probe only the surface of a drug sample, so they couldn’t be used for a pill that’s still in its packaging or for medicine inside a capsule. And these optical methods could be fooled by pills manufactured with coatings that contain the features of the correct drug.
That said, we readily admit that NQR has some special challenges. The biggest are its inherently low signal levels and poor signal-to-noise ratios. Those same issues are what stymied earlier applications of NQR to explosives detection. A related problem is external RF interference, created, say, by an AM radio station or by a noisy switching power supply in nearby equipment. And finally, the NQR sample coil is normally attached to capacitors in what’s known as a tuned circuit, so that power can be applied to it effectively during transmission and to ensure that it is sensitive to the appropriate spectral band during the reception interval. (A tuned circuit naturally resonates at a particular frequency, allowing it to absorb energy at that frequency—just as a wine glass does when a singer hits a note that shatters it.) Tuning the coil manually to measure each new NQR spectral line would be a difficult and time-consuming process.
Over the past four years, our research groups have overcome all of these challenges while working to develop a sensitive, low-cost, and reliable NQR-based drug-authentication system. We addressed the issue of low sensitivity with a technique called polarization transfer, which was explored a decade or more ago in the application of NQR to explosives detection. For this, the sample is briefly placed in a magnetic field of about half a tesla, generated by a permanent magnet. The sample is then rapidly removed from the magnet using a motorized actuator. Some complicated physics then ensues between hydrogen nuclei in the specimen (which respond to magnetic fields) and nearby NQR-sensitive nuclei. This process typically increases the NQR signal amplitude by a factor of 5 to 10. At that level, very small samples with only about a gram of active ingredient can be reliably tested within a minute or less. By increasing the magnetic field to 2 T, which can be done readily with rare-earth magnets, we should be able to get even better results, although the stronger magnet would raise manufacturing costs somewhat.
We dealt with RF interference in a few different ways. The first was simply to build proper RF shielding into our equipment. Our prototype also uses adaptive noise cancellation—similar to what’s done by noise-canceling headphones—as well as signal-processing methods that can separate the signal from the interference.
To address the awkwardness of having to manually tune the coil for a given frequency band, we used digitally programmable tuning circuits, as have been used in commercial NQR gear in the past. These circuits contain capacitor arrays and miniature relays that are digitally controlled, thus allowing a computer to alter the coil’s resonant frequency on command.
We continue to improve our NQR-based drug-authentication system and are also exploring approaches that combine these measurements with near-infrared optical spectroscopy. We hope that such technology might one day see widespread application at hospitals, health clinics, neighborhood pharmacies, maybe even inside some people’s homes. Being able to test pharmaceuticals quickly and reliably would go a long way toward improving the integrity and security of the supply chain for essential medicines.
This article appears in the September 2019 print issue as “Countering Counterfeit Drugs.”
About the Author
Swarup Bhunia is a professor of electrical and computering engineering at the University of Florida, where he leads the Nanoscape Research Laboratory. Soumyajit Mandal is an assistant professor of electrical engineering and computer science at Case Western Reserve University, where he oversees the Integrated Circuits and Sensor Physics Lab.