ALV 196 Pill: Uses, Dosage, Side Effects, Addiction
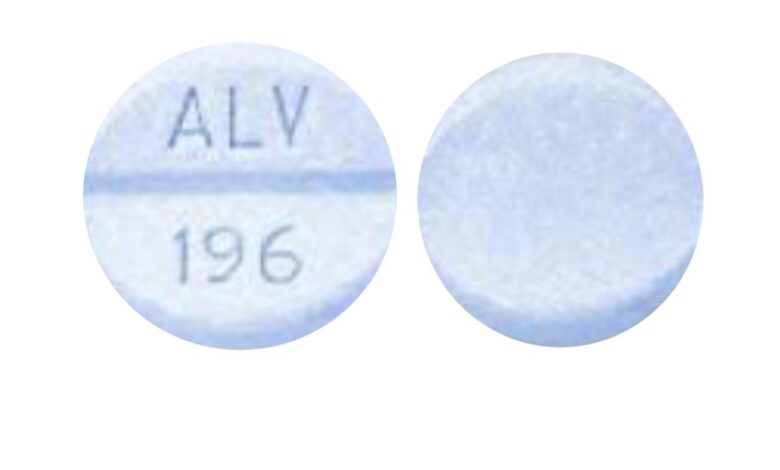
The blue round pill with the imprint ALV 196 has been identified as Acetaminophen and Oxycodone Hydrochloride 325 mg / 5 mg supplied by Alvogen, Inc.. This combination medication is used to help relieve moderate to severe pain and when other pain medicines did not work well enough or cannot be tolerated. ALV 196 pill provides relief for moderate to severe pain and lasts up to 3 to 5 hours depending on the formulation.
Acetaminophen is used to relieve pain and reduce fever in patients. It does not become habit-forming when taken for a long time. But acetaminophen may cause other unwanted effects when taken in large doses, including liver damage.
Oxycodone belongs to the group of medicines called narcotic analgesics (pain medicines). It acts on the central nervous system (CNS) to relieve pain. When oxycodone is used for a long time, it may become habit-forming, causing mental or physical dependence. However, people who have continuing pain should not let the fear of dependence keep them from using narcotics to relieve their pain. Mental dependence (addiction) is not likely to occur when narcotics are used for this purpose. Physical dependence may lead to withdrawal side effects if treatment is stopped suddenly. However, severe withdrawal side effects can usually be prevented by gradually reducing the dose over a period of time before treatment is stopped completely.
How to use ALV 196 pill
Take this medicine only as directed by your doctor. Do not take more of it, do not take it more often, and do not take it for a longer time than your doctor ordered. This is especially important for elderly patients, who may be more sensitive to the effects of pain medicines. If too much of this medicine is taken for a long time, it may become habit-forming and cause mental or physical dependence. Also, large amounts of acetaminophen may cause liver damage if taken for a long time.
You may take this medicine with or without food.
This combination medicine contains acetaminophen (Tylenol®). Carefully check the labels of all other medicines you are using, because they may also contain acetaminophen. It is not safe to use more than 4 grams (4,000 milligrams) of acetaminophen in one day (24 hours).
The dose of this medicine will be different for different patients. Follow your doctor’s orders or the directions on the label. The following information includes only the average doses of this medicine. If your dose is different, do not change it unless your doctor tells you to do so.
What are the side effects of ALV 196 pill?
Along with its needed effects, this medicine may cause some unwanted effects. Although not all of these side effects may occur, if they do occur they may need medical attention.
Check with your doctor immediately if any of the following side effects occur:
More common
• Black, tarry stools
• chills
• dark urine
• dizziness
• fever
• headache
• itching, skin rash
• light-colored stools
• loss of appetite
• nausea
• stomach pain
• unpleasant breath odor
• unusual tiredness or weakness
• vomiting of blood
• yellow eyes or skin
Rare
• Cough
• fever with or without chills
• general feeling of tiredness or weakness
• hoarseness
• lower back or side pain
• painful or difficult urination
• sore throat
• sores, ulcers, or white spots on the lips or in the mouth
• unusual bleeding or bruising
Incidence not known
• Back, leg, or stomach pains
• bleeding gums
• bloating
• blood in the urine or stools
• blue lips and fingernails
• blurred vision
• burning, crawling, itching, numbness, prickling, “pins and needles”, or tingling feelings
• chest pain or discomfort
• cloudy urine
• clumsiness
• confusion
• constipation
• coughing that sometimes produces a pink frothy sputum
• decreased awareness or responsiveness
• decreased frequency or amount of urine
• difficult, fast, noisy breathing
• difficulty in passing urine (dribbling)
• difficulty with swallowing
• dizziness, faintness, or lightheadedness when getting up suddenly from a lying or sitting position
• drowsiness
• dry mouth
• extremely shallow or slow breathing
• fainting
• fast or deep breathing
• fast, slow, irregular, pounding, or racing heartbeat
• feeling of warmth
• general body swelling
• hives or welts
• increased sweating
• increased thirst
• indigestion
• large, hive-like swelling on the face, eyelids, lips, tongue, throat, hands, legs, feet, or genitals
• lightheadedness
• muscle aches, tremors, or weakness
• nervousness
• nosebleeds
• pains in the stomach, side, or abdomen, possibly radiating to the back
• pale skin
• pinpoint red spots on the skin
• pounding in the ears
• puffiness or swelling of the eyelids or around the eyes, face, lips, or tongue
• rapid, deep or shallow breathing
• redness of the face, neck, arms, and occasionally, upper chest
• restlessness
• seizures
• severe constipation
• severe sleepiness
• severe vomiting
• skin blisters
• sleepiness
• stomach cramps
• sunken eyes
• sweating
• swelling of the face, fingers, lower legs, or ankles
• thirst
• tightness in the chest
• tiredness
• trouble breathing
• vomiting
• weakness or heaviness of the legs
• weight gain
Get emergency help immediately if any of the following symptoms of overdose occur:
Symptoms of overdose
• Bluish lips or skin
• change in consciousness
• cold, clammy skin
• extreme sleepiness
• general feeling of discomfort or illness
• loss of consciousness
• low blood pressure or pulse
• slow breathing
• unconsciousness
Some side effects may occur that usually do not need medical attention. These side effects may go away during treatment as your body adjusts to the medicine. Also, your health care professional may be able to tell you about ways to prevent or reduce some of these side effects. Check with your health care professional if any of the side effects continue or are bothersome or if you have any questions about them.
ALV 196 Pill Safety Information
It is very important that your doctor check your progress while you are taking this medicine, especially within the first 24 to 72 hours of treatment. This will allow your doctor to see if the medicine is working properly and to decide if you should continue to take it. Blood and urine tests may be needed to check for unwanted effects.
It is against the law and dangerous for anyone else to use your medicine. Keep your unused medicine in a safe and secure place. People who are addicted to drugs might want to steal it.
This medicine will add to the effects of alcohol and other CNS depressants (medicines that can make you drowsy or less alert). Some examples of CNS depressants are antihistamines or medicine for allergies or colds, sedatives, tranquilizers, or sleeping medicine, other prescription pain medicine or narcotics, medicine for seizures or barbiturates, muscle relaxants, or anesthetics, including some dental anesthetics. Also, there may be a greater risk of liver damage if you drink three or more alcoholic beverages while you are taking acetaminophen. Do not drink alcoholic beverages, and check with your doctor before taking any of these medicines while you are using this medicine.
This medicine may be habit-forming. If you feel that the medicine is not working as well, do not use more than your prescribed dose. Call your doctor for instructions.
Do not use this medicine if you are using or have used an MAO inhibitor (eg, isocarboxazid [Marplan®], linezolid [Zyvox®], phenelzine [Nardil®], selegiline [Eldepryl®], tranylcypromine [Parnate®]) within the past 14 days. Using these medicine together may cause serious unwanted effects.
This medicine may cause sleep-related breathing problems (eg, sleep apnea, sleep-related hypoxemia). Your doctor may decrease your dose if you have sleep apnea (stop breathing for short periods during sleep) while using this medicine.
Dizziness, lightheadedness, or fainting may occur when you get up suddenly from a lying or sitting position. Getting up slowly may help lessen this problem. Also, lying down for a while may relieve the dizziness or lightheadedness.
This medicine may make you dizzy, drowsy, or lightheaded. Do not drive or do anything else that could be dangerous until you know how this medicine affects you.
Check with your doctor right away if you have pain or tenderness in the upper stomach, pale stools, dark urine, loss of appetite, nausea, unusual tiredness or weakness, or yellow eyes or skin. These could be symptoms of a serious liver problem.
Serious skin reactions can occur with this medicine. Check with your doctor right away if you have blistering, peeling, or loosening of the skin, red skin lesions, severe acne or skin rash, sores or ulcers on the skin, or fever or chills while you are using this medicine.
This medicine may cause serious allergic reactions, including anaphylaxis, which can be life-threatening and requires immediate medical attention. Call your doctor right away if you have a rash, itching, hoarseness, trouble breathing, trouble swallowing, or any swelling of your hands, face, or mouth while you are using this medicine.
Using narcotics for a long time can cause severe constipation. To prevent this, your doctor may direct you to take laxatives, drink a lot of fluids, or increase the amount of fiber in your diet. Be sure to follow the directions carefully, because continuing constipation can lead to more serious problems.
If you have been using this medicine regularly for several weeks or longer, do not change your dose or suddenly stop using it without checking with your doctor. Your doctor may want you to gradually reduce the amount you are using before stopping it completely. This may help prevent worsening of your condition and reduce the possibility of withdrawal symptoms, such as stomach cramps, anxiety, fever, irritability, nausea, restlessness, runny nose, sweating, tremors, or trouble with sleeping.
Using this medicine while you are pregnant may cause serious unwanted effects, including neonatal withdrawal syndrome in your newborn baby. Tell your doctor right away if you think you are pregnant or if you plan to become pregnant while using this medicine.
For nursing mothers:
- Talk to your doctor if you have any questions about taking oxycodone or about how this medicine may affect your baby.
- Call your doctor if you become extremely tired and have difficulty caring for your baby.
- Your baby should generally nurse every 2 to 3 hours and should not sleep for more than 4 hours at a time.
- Check with your doctor or hospital emergency room immediately if your baby shows signs of increased sleepiness (more than usual), difficulty breastfeeding, difficulty breathing, or limpness. These may be symptoms of an overdose and need immediate medical attention.
Before you have any medical tests, tell the medical doctor in charge that you are taking this medicine. The results of some tests may be affected by this medicine.
Check with your doctor right away if you have anxiety, restlessness, a fast heartbeat, fever, sweating, muscle spasms, twitching, nausea, vomiting, diarrhea, or see or hear things that are not there. These may be symptoms of a serious condition called serotonin syndrome. Your risk may be higher if you also take certain other medicines that affect serotonin levels in your body.
Using too much of this medicine may reduce infertility (unable to have children). Talk with your doctor before using this medicine if you plan to have children.
Do not take other medicines unless they have been discussed with your doctor. This includes prescription or nonprescription (over-the-counter [OTC]) medicines and herbal or vitamin supplements.