Dozol Oral Solution: Uses, Dosage, Side Effects, Warnings
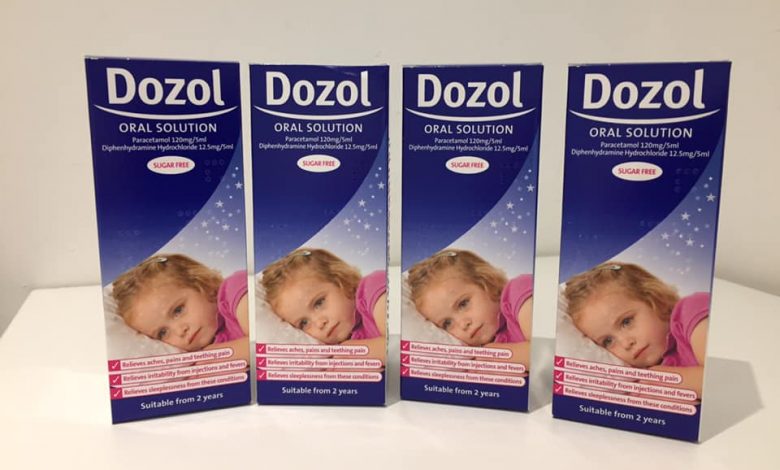
Dozol Oral Solution is a combination medicine containing paracetamol and diphenhydramine as active ingredients in a caramel-flavored sugar-free oral solution. Dozol Oral Solution is used to treat occasional insomnia associated with minor aches and pains. Dozol Oral Solution is not for use in treating sleeplessness without pain, or sleep problems that occur often.
Dozol Oral Solution is indicated in children aged over 2 years in the relief of teething pains, irritability associated with injections or feverishness, aches or pains, sleeplessness associated with the above conditions.
Warnings
Use this medicine exactly as directed. An overdose of paracetamol can damage your child’s liver or cause death. Taking too much diphenhydramine can lead to serious heart problems, seizures, coma, or death.
Do not use this medicine to make a child sleepy. Ask a doctor or pharmacist before using any other medicine that may contain paracetamol or diphenhydramine. Taking too much of either medicine can lead to a fatal overdose. Drinking alcohol may increase your risk of liver damage while taking paracetamol.
Stop using this medicine and call your doctor right away if you notice skin redness or a rash that spreads and causes blistering and peeling.
How to use Dozol Oral Solution?
The recommended dose of Dozol Oral Solution is as follows:
Children 2 – 6 years: give 5-10ml (3 to 4 times daily).
Children over 6 years: give 10-20ml (3 times daily).
Never give more than the recommended dosage in any 24 hours and leave at least 4 hours between each dose. Dozol contains Paracetamol, which is a painkiller suitable for children between the ages of 2 and 12 years. This medication helps in relieving pain from such ailments as toothache, sore throats, earaches colds and flu, and general aches and pains. This medicine also reduces high temperatures and fevers associated with infections such as chickenpox, whooping cough, measles, and mumps. It also contains diphenhydramine hydrochloride which helps with sleeplessness associated with the above illnesses.
What happens if I miss a dose of Dozol Oral Solution?
Since Dozol Oral Solution is used when needed, you may not be on a dosing schedule. Skip any missed dose if it’s almost time for your next dose. Do not use two doses at one time.
What happens if I overdose?
Seek emergency medical attention or call the Poison Help line at 1-800-222-1222. An overdose can be fatal or cause liver damage.
Overdose symptoms may include loss of appetite, vomiting, weakness, confusion, ringing in your ears, upper stomach pain, dark urine, no urination, very dry eyes and mouth, yellowing of your skin or eyes, dilated pupils, fast heartbeats, tremor, agitation, hallucinations, or seizure.
If you are pregnant or breastfeeding.
If you are pregnant or trying to become pregnant, please consult your doctor before purchasing or taking Dozol Oral Solution.
Side effects of Dozol Oral Solution
Common side effects of Dozol oral include:
- Blurred vision
- Difficulty passing urine
- Drowsiness
- Dry mouth nose and throat
- Headache
- Phlegm on the chest
- Upset stomach
Other side effects
Paracetamol. Side effects of paracetamol are rare and usually mild, although hematological reactions have been reported. Skin rashes and hypersensitivity reactions occur occasionally.
Overdosage with paracetamol, if left untreated, can result in severe, sometimes fatal liver damage and rarely, acute renal tubular necrosis. Adverse events from historical clinical trial data are both infrequent and from small patient exposure. Accordingly, events reported from extensive post-marketing experience at therapeutic/labeled dose and considered attributable are listed below by system organ class and frequency. As the adverse reactions identified from post-marketing use are reported voluntarily from a population of uncertain size, the frequency is not known but is likely to be very rare.
Blood and lymphatic system disorders: Thrombocytopenia.
Immune system disorders: Anaphylaxis, cutaneous hypersensitivity reactions including skin rashes, angioedema, and Stevens-Johnson syndrome.
Respiratory, thoracic, and mediastinal disorders: Bronchospasm in patients sensitive to aspirin and other NSAIDs.
Hepatobiliary disorders: Hepatic dysfunction.
Diphenhydramine. Adverse reactions that have been observed in clinical trials and which are considered to be common or very common are listed below. The frequency of other adverse reactions identified during post-marketing use is not known but these reactions are likely to be uncommon or rare.
Central nervous system (CNS) effects. CNS depressive effects of diphenhydramine hydrochloride include sedation and impaired performance (impaired driving performance, poor work performance, incoordination, reduced motor skills, and impaired information processing).
Performance may be impaired in the absence of sedation and may persist the morning after a nighttime dose. CNS stimulatory effects of diphenhydramine may include anxiety, hallucinations, appetite stimulation, muscle dyskinesias, and activation of epileptogenic foci. High doses of diphenhydramine may cause nervousness, tremor, insomnia, agitation, and irritability.
Anticholinergic effects. Side effects of diphenhydramine associated with cholinergic blockage include dryness of the eyes, mouth, and nose, blurred vision, urinary hesitancy and retention, constipation, and tachycardia.